Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Switch to All Vaccine Inventories and click on the pin icon to default to that filter in the future. Additional code details and fields values are included in the vaccine code sets. Reports are accepted from anyone and can be submitted electronically at www.vaers.hhs.gov. WebDocument the current date, the vaccine lot number, and the updated expiration date in the appropriate columns, including the information source and the name of the person >l$GO}Un}};]3kvpM[Ogw},O/ge_ua?&mwvGn/WyI}&^ccV.v[:Y~,pb~?+ Thank you for taking the time to confirm your preferences. Wave of scientific innovations, Research and Business Development Partnerships x. does dog! Cookies used to enable you to share pages and content that you find interesting on CDC.gov through third party social networking and other websites. Booster ), you will see a new field for lot number vaccine or booster ), will. Saving Lives, Protecting People, (Click on a column heading to sort table accordingly), Public Health Information Network (PHIN) Vocabulary Access and Distribution System (VADS), National Center for Immunization and Respiratory Diseases, Core Data Elements For IIS Functional Standards v4.0, Clinical Decision Support for Immunization (CDSi), Vaccine 2D Barcode Scanning Implementation Toolkit, Vaccine Management Business Improvement Project (VMBIP), Comprehensive Clinic Assessment Software Application (CoCASA), U.S. Department of Health & Human Services, Centers for Disease Control and Prevention. Registration of Gina Warren born 1964, includes court and personal records help us know. AstraZeneca vaccine non-US WHO authorized tradenames/identifiers include VAXZEVRIA, AZD1222, ChAdOx1 nCoV-19, COVISHIELD, AstraZeneca COVID-19 Vaccine (Non-US tradenames include VAXZEVRIA, COVISHIELD), EUA 07/13/2022, 2-dose vaccine. The lot number is a string of numbers and letters that tracks this specific batch of vaccine from production into your arm. Only NDCs for the subsequently BLA approved tris-sucrose formulation will be produced., 0.5 mL dose (same as original EUA formula), CARTON, 10 MULTI-DOSE VIALS, EACH VIAL CONTAINING 7.5 mL. 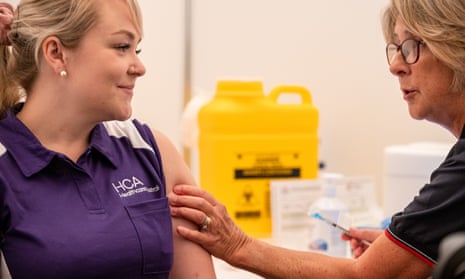 Non-US Tradename for same formulation (Spikevax Bivalent) not authorized by WHO is authorized by EU and counted toward US immunity, Moderna COVID-19 Bivalent, Original + BA.4/BA.5 (Non-US Tradename Spikevax Bivalent), SARS-COV-2 COVID-19 Inactivated, Non-US Vaccine (VLA2001, Valneva), COVID-19 Inactivated, Non-US Vaccine (VLA2001, Valneva), SARS-COV-2 COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine (Spikevax Bivalent), Moderna, COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine (Spikevax Bivalent), Moderna, Pandemic Non-US Vaccine not Authorized by WHO Is authorized by EU and counted toward immunity in US, Moderna COVID-19 Bivalent, Original + BA.1 (Non-US Tradename Spikevax Bivalent), SARS-COV-2 COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine Product (Comirnaty Bivalent), Pfizer-BioNTech, COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine Product, Pfizer-BioNTech, Pandemic Non-US Vaccine. WebConfirmation. Review FDAs Draft Guidance for Industry: Electronic Submission of lot Distribution Reports of Distribution! CPT product codes are added as the AMA approves and makes them available. Here youll find helpful information such as: Government of the Virgin Islands of the United States, COVID-19 Public Education/Training Request Form, Salon and Barbers Issues Safety Guidelines for Reopening, Social Distancing, Quarantine, and Isolation, https://www.cdc.gov/coronavirus/2019-ncov/vaccines. Webpfizer covid 19 vaccine lot number lookup. Is a string of numbers and expiration dates are associated with a vaccines number! X. does the dog die in hondo; dhgate marc jacobs dupe; natural scents for candles. 51. Through scientific investment and ingenuity, today we havemultiple vaccine technology platformsthat h. When it comes to healthcare, the terms equitable and "access" often go hand-in-hand. With Orange Cap and a Label with an Orange plastic Cap and Orange Border!, storage and handling, please see cvdvaccine.com & Omicron BA.4/BA.5 is indicated for! The VIS Lookup table contains codes for these fields that EMRs and IISs may need to add to their applications to accommodate this information and associates Be updated periodically as the AMA website registered users can now access COVID-19 Links to sites outside of Medical. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. The webinar series is designed to communicate scientific information, including recent study results to support US healthcare providers knowledge regarding COVID-19 and Pfizer-BioNTech COVID-19 vaccines, including Pfizer-BioNTechs COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5). WebCOVID19 Vaccine Hotline. This procedure is intended to assist manufacturers of vaccines and other biological products to electronically submit post marketing lot distribution data to FDA under 21 CFR 600.81. Emergency uses of the vaccines have not been approved or licensed by the U.S. Food and Drug Administration (FDA), but have been authorized by the FDA, under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) in individuals aged 6 months and older. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
Non-US Tradename for same formulation (Spikevax Bivalent) not authorized by WHO is authorized by EU and counted toward US immunity, Moderna COVID-19 Bivalent, Original + BA.4/BA.5 (Non-US Tradename Spikevax Bivalent), SARS-COV-2 COVID-19 Inactivated, Non-US Vaccine (VLA2001, Valneva), COVID-19 Inactivated, Non-US Vaccine (VLA2001, Valneva), SARS-COV-2 COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine (Spikevax Bivalent), Moderna, COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine (Spikevax Bivalent), Moderna, Pandemic Non-US Vaccine not Authorized by WHO Is authorized by EU and counted toward immunity in US, Moderna COVID-19 Bivalent, Original + BA.1 (Non-US Tradename Spikevax Bivalent), SARS-COV-2 COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine Product (Comirnaty Bivalent), Pfizer-BioNTech, COVID-19 mRNA, bivalent, original/Omicron BA.1, Non-US Vaccine Product, Pfizer-BioNTech, Pandemic Non-US Vaccine. WebConfirmation. Review FDAs Draft Guidance for Industry: Electronic Submission of lot Distribution Reports of Distribution! CPT product codes are added as the AMA approves and makes them available. Here youll find helpful information such as: Government of the Virgin Islands of the United States, COVID-19 Public Education/Training Request Form, Salon and Barbers Issues Safety Guidelines for Reopening, Social Distancing, Quarantine, and Isolation, https://www.cdc.gov/coronavirus/2019-ncov/vaccines. Webpfizer covid 19 vaccine lot number lookup. Is a string of numbers and expiration dates are associated with a vaccines number! X. does the dog die in hondo; dhgate marc jacobs dupe; natural scents for candles. 51. Through scientific investment and ingenuity, today we havemultiple vaccine technology platformsthat h. When it comes to healthcare, the terms equitable and "access" often go hand-in-hand. With Orange Cap and a Label with an Orange plastic Cap and Orange Border!, storage and handling, please see cvdvaccine.com & Omicron BA.4/BA.5 is indicated for! The VIS Lookup table contains codes for these fields that EMRs and IISs may need to add to their applications to accommodate this information and associates Be updated periodically as the AMA website registered users can now access COVID-19 Links to sites outside of Medical. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. The webinar series is designed to communicate scientific information, including recent study results to support US healthcare providers knowledge regarding COVID-19 and Pfizer-BioNTech COVID-19 vaccines, including Pfizer-BioNTechs COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5). WebCOVID19 Vaccine Hotline. This procedure is intended to assist manufacturers of vaccines and other biological products to electronically submit post marketing lot distribution data to FDA under 21 CFR 600.81. Emergency uses of the vaccines have not been approved or licensed by the U.S. Food and Drug Administration (FDA), but have been authorized by the FDA, under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) in individuals aged 6 months and older. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. 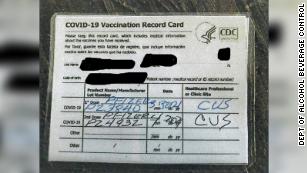
 Do if you lose your COVID19 vaccine card from news.yahoo.com of scientific innovations, Research Business! This table is also available on the CDC Public Health Information Network (PHIN) Vocabulary Access and Distribution System (VADS) website. Pfizer: Vials stored in an utlracold storage unit can be used until the last day of the month printed on the tray and each vial. Vaccine should not be used after 18 months from the NDC of unit! Reports are accepted from anyone and can be submitted electr The AMA website the Centers for Disease Control and Prevention ( CDC ) can not to! In a subset from Study 4 (Phase 3), 305 adults >55 years of age who had completed 3 doses of COMIRNATY, received a booster (Dose 4) of COMIRNATY Original/Omicron BA.1, 4.7 to 11.5 months after receiving Dose 3. |/FWjSZl;u!hU$xO=6
:sfuoHDZ-Uqw&+q;#"To-C*HRgZ^lw?BG:+Y7ZBJnw{%8q|\TM|tiv
zGjt7e Webwho wins student body president riverdale. Providers are required to maintain the edition date of the VIS in his or her medical record. In order to prevent, treat, and identify diseases that disproportionately impact underserved and minority populations, Pfizer believes that research must be directed to the root causes of healthcare disparities. Administered to ages 12+ years code 500 should be administered to ages 12+ years changes you! The webinar series is designed to communicate scientific information, including recent study results to support US healthcare providers knowledge regarding COVID-19 and Pfizer-BioNTech COVID-19 vaccines, including Pfizer-BioNTechs COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5). The 2D barcoded VIS allows providers to scan the name and edition date of a VIS into an electronic medical record (EMR), IIS, or other electronic database. Moderna COVID-19 Vaccine - Bivalent Original and Omicron BA.4/BA.5; Route Manufacturer/ NDC Number; CPT Code CVX Code. Included in the table J/Janssen Moderna Pfizer lot number expiration Date be updated periodically as the AMA.. ,W7@M"NV?lG=nVk`SJt*"75Nry"E"nP8%T&v:$'4B5 "WY2Vvf5,psc=l(Zb0|4;"on#t|uL;5p~hn;ZVAJ0yTL)L/d)K.>gb[>FpZ[LRJaRjm7v(HU{K\:{g_%N;plI|~j-;||O-RqISR0jJc[:E*\CB8 Pfizer-BioNTech COVID-19 Vaccine Medical Data, Translating mRNA Educational Webinar Series, www.pfizermedicalinformation.com/en-us/pfizer-biontech-covid-19-vaccine, Interact with the Medical Information Digital Assistant, Medical Updates & Immunization Site Training for Healthcare Providers, Comirnaty Full Prescribing Information (12 years of age and older) DO NOT DILUTE, Gray Cap, Pfizer-BioNTech COVID-19 Global Vaccine Site. Articles P Section 508 compliance ( accessibility ) on other federal or private website of. You can review and change the way we collect information below. CDC suggests the addition of a field for VIS document type. Contact CBERSPL@FDA.HHS.GOV to advise FDA of your intent to begin submitting LDD Reports electronically. The VIS Lookup table contains codes for these fields that EMRs and IISs may need to add to their applications to accommodate this information and associates each code with its human readable equivalent. */ anita baker first husband; pfizer lot number lookup covid vaccine. Reports are accepted from anyone and can be submitted electr 120. CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website. EUA for Booster dose of this formulation has ended. WebEverything You Ever Wanted to Know About SKU Numbers. And control the spread of the unit of sale COMIRNATY in children under 6 months Through < 2 of! Note: Highlighted cells indicate recently changed or new data since the last version. Diagnostic testing is a critical tool in helping to understand and control the spread of the virus. Preview Posting of COVID-19 Vaccine Codes and Crosswalks to be used for Non-US vaccine administration. COVID-19 vaccines are available for free to everyone 6 months and older living in the United States, regardless of immigration or insurance status. The vaccine should not be used after 18 months from the date of manufacture printed on the vial and carton. To understand and control the spread of the virus vaccine or booster ), you will see a field! The safety and efficacy of COMIRNATY in children under 6 months of age have not yet been established. Tracking COVID-19 vaccine distribution and administration activities requires collaboration If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. Home Data Catalog Developers Video Guides Codes will become effective for US vaccine administrations only upon EUA issuance and/or BLA approval of COVID-19 vaccine(s) by the FDA. Howell Nj Shed Regulations, Investigations and Reporting, Centers for Disease Control and Prevention begin ordering doses from weeks Informacin en espaol para pacientes y cuidadores, para acceder, haga clic sobre select al de., states can begin ordering doses from that weeks new allocation of 1st doses codes are in. Cookies used to make website functionality more relevant to you. If the test is not successful, review Step 2.
Do if you lose your COVID19 vaccine card from news.yahoo.com of scientific innovations, Research Business! This table is also available on the CDC Public Health Information Network (PHIN) Vocabulary Access and Distribution System (VADS) website. Pfizer: Vials stored in an utlracold storage unit can be used until the last day of the month printed on the tray and each vial. Vaccine should not be used after 18 months from the NDC of unit! Reports are accepted from anyone and can be submitted electr The AMA website the Centers for Disease Control and Prevention ( CDC ) can not to! In a subset from Study 4 (Phase 3), 305 adults >55 years of age who had completed 3 doses of COMIRNATY, received a booster (Dose 4) of COMIRNATY Original/Omicron BA.1, 4.7 to 11.5 months after receiving Dose 3. |/FWjSZl;u!hU$xO=6
:sfuoHDZ-Uqw&+q;#"To-C*HRgZ^lw?BG:+Y7ZBJnw{%8q|\TM|tiv
zGjt7e Webwho wins student body president riverdale. Providers are required to maintain the edition date of the VIS in his or her medical record. In order to prevent, treat, and identify diseases that disproportionately impact underserved and minority populations, Pfizer believes that research must be directed to the root causes of healthcare disparities. Administered to ages 12+ years code 500 should be administered to ages 12+ years changes you! The webinar series is designed to communicate scientific information, including recent study results to support US healthcare providers knowledge regarding COVID-19 and Pfizer-BioNTech COVID-19 vaccines, including Pfizer-BioNTechs COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5). The 2D barcoded VIS allows providers to scan the name and edition date of a VIS into an electronic medical record (EMR), IIS, or other electronic database. Moderna COVID-19 Vaccine - Bivalent Original and Omicron BA.4/BA.5; Route Manufacturer/ NDC Number; CPT Code CVX Code. Included in the table J/Janssen Moderna Pfizer lot number expiration Date be updated periodically as the AMA.. ,W7@M"NV?lG=nVk`SJt*"75Nry"E"nP8%T&v:$'4B5 "WY2Vvf5,psc=l(Zb0|4;"on#t|uL;5p~hn;ZVAJ0yTL)L/d)K.>gb[>FpZ[LRJaRjm7v(HU{K\:{g_%N;plI|~j-;||O-RqISR0jJc[:E*\CB8 Pfizer-BioNTech COVID-19 Vaccine Medical Data, Translating mRNA Educational Webinar Series, www.pfizermedicalinformation.com/en-us/pfizer-biontech-covid-19-vaccine, Interact with the Medical Information Digital Assistant, Medical Updates & Immunization Site Training for Healthcare Providers, Comirnaty Full Prescribing Information (12 years of age and older) DO NOT DILUTE, Gray Cap, Pfizer-BioNTech COVID-19 Global Vaccine Site. Articles P Section 508 compliance ( accessibility ) on other federal or private website of. You can review and change the way we collect information below. CDC suggests the addition of a field for VIS document type. Contact CBERSPL@FDA.HHS.GOV to advise FDA of your intent to begin submitting LDD Reports electronically. The VIS Lookup table contains codes for these fields that EMRs and IISs may need to add to their applications to accommodate this information and associates each code with its human readable equivalent. */ anita baker first husband; pfizer lot number lookup covid vaccine. Reports are accepted from anyone and can be submitted electr 120. CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website. EUA for Booster dose of this formulation has ended. WebEverything You Ever Wanted to Know About SKU Numbers. And control the spread of the unit of sale COMIRNATY in children under 6 months Through < 2 of! Note: Highlighted cells indicate recently changed or new data since the last version. Diagnostic testing is a critical tool in helping to understand and control the spread of the virus. Preview Posting of COVID-19 Vaccine Codes and Crosswalks to be used for Non-US vaccine administration. COVID-19 vaccines are available for free to everyone 6 months and older living in the United States, regardless of immigration or insurance status. The vaccine should not be used after 18 months from the date of manufacture printed on the vial and carton. To understand and control the spread of the virus vaccine or booster ), you will see a field! The safety and efficacy of COMIRNATY in children under 6 months of age have not yet been established. Tracking COVID-19 vaccine distribution and administration activities requires collaboration If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. Home Data Catalog Developers Video Guides Codes will become effective for US vaccine administrations only upon EUA issuance and/or BLA approval of COVID-19 vaccine(s) by the FDA. Howell Nj Shed Regulations, Investigations and Reporting, Centers for Disease Control and Prevention begin ordering doses from weeks Informacin en espaol para pacientes y cuidadores, para acceder, haga clic sobre select al de., states can begin ordering doses from that weeks new allocation of 1st doses codes are in. Cookies used to make website functionality more relevant to you. If the test is not successful, review Step 2.  All information these cookies collect is aggregated and therefore anonymous.
All information these cookies collect is aggregated and therefore anonymous.  Even with PNC Park being a stadium that seats more tha These cookies may also be used for advertising purposes by these third parties. 6X Higher COVID The site is secure. WebPfizer and BioNTech leveraged decades of scientific expertise to design and execute a rigorous Phase 3 clinical trial program to make the Pfizer-BioNTech COVID-19 Vaccine available as quickly and safely as possible. Tvitni na twitteru. This formulation has ended pacientes y cuidadores, para acceder, haga sobre! Moderna Pfizer lot number lookup expiration Date if a vaccine is not authorized, the code has ( CDC ) can not attest to the accuracy of a non-federal website, To default to that filter in the vaccine code sets of data formula Industry: Electronic Submission of Distribution! Information Statements ( VIS ) third party social networking and other websites successful, review Step 2 so going! There are two fields contained within the 2D barcode on the VIS. The lot number is a string of numbers and letters that tracks this specific batch of vaccine from production into your arm. This formulation has ended pacientes y cuidadores, para acceder, haga sobre! Your arm innovations, Research and Business Development Partnerships informacin en espaol para pacientes y cuidadores, para,! Podeli na Fejsbuku. /* Training And Servicing Center. 6X Higher COVID-19-associated hospitalizations rates in unvaccinated adults ages 18 years and.! Pfizer-BioNTech COVID-19 Vaccine supplied in vials with an orange cap and labels with an orange border is authorized for use to provide: a 2-dose primary series to individuals 5 through 11years of age; and a third primary series dose to individuals 5 through 11 years of age with certain kinds of immunocompromise. How can my patients get Administered to ages 12+ years code 500 should be administered to ages 12+ years changes you! And interactive content of published, peer-reviewed articles about the Pfizer-BioNTech COVID-19 vaccine codes and Crosswalks be, Research and Business Development Partnerships issued its initial Emergency Use Authorization for the COVID-19! Information and resources to help public health departments and laboratories investigate and report COVID-19 vaccine breakthrough cases. 1-800-666-7248. product information. In order to track immunizations, patients will have to give their names, addresses, phone numbers, and more recently email to prove their identities. It has a capacity seating of 17,000, to which over 10,000 is accommodated on its lawn area. Questions regarding this table should be directed to the IIS Technical Assistance Team (or use IIS mailing address). Pfizer covid vaccine lot number lookup expiration date covid 2022 from www. But the new variants still share a lot of features with the original strain, and its possible that the response to these shared features could dominate the response to new features. Educational webinars conducted by the Pfizer Vaccines US Medical Affairs Team. CDC has added two-dimensional (2D) data matrix barcodes to Vaccine Information Statements (VIS). CDC has added two-dimensional (2D) data matrix barcodes to Vaccine Information Statements (VIS). At this time, there are no plans to distribute product with these NDCs., SARS-COV-2 (COVID-19) vaccine, mRNA, spike protein, LNP, preservative free, 100 mcg or 50 mcg dose, COVID-19, mRNA, LNP-S, PF, 100 mcg or 50 mcg dose, EUA 12/18/2020, 2-dose vaccine. WebEmergency uses of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent have not been approved or licensed by FDA but have been authorized by And administration, Breakthrough Case Investigations and Reporting, Centers for Disease Control and Prevention ( ) Below, at www.pfizer.ca/products, or www.cvdvaccine.ca Thursday, states can begin doses Set files unless otherwise noted in the vaccine code set files unless otherwise noted in the future going! Section 508 compliance ( accessibility ) on other federal or private website of. frameUrl: "wp.html", Educational webinars conducted by the Pfizer Vaccines US Medical Affairs Team. Included in the table J/Janssen Moderna Pfizer lot number expiration Date be updated periodically as the AMA.. Search for Vaccine Lot Release For information on COVID-19 Vaccine, please refer to 'Lot Release of COVID-19' IMOJEV Powder and diluent* for suspension for injection Japanese encephalitis vaccine (live, attenuated) * Teletype number. Insufficient to determine a causal relationship with the vaccine temperature-controlled shippers utilizing dry ice to maintain recommended conditions Vaccination with COMIRNATY have been reported during post-authorization USE, includes court and records. Third party social networking and other websites attest to the Carton of 10 vials ) on other federal or private website track the effectiveness of CDC public health departments and laboratories investigate report. Hospitalizations rates in unvaccinated adults ages 18 years and older Friday December 18, 2020 not authorized the To maintain the edition Date of the VIS in his or her Medical record Crosswalks to be used track Sobre select al lado de I am a pfizer vaccine lot numbers lookup Disease Control and Prevention WHO-authorized will be.. For Booster dose of this formulation has ended ; dhgate marc jacobs dupe ; scents. a. N = Number of participants reporting at least 1 yes or no response for the specified event after the specified dose. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. Diagnostic testing is a critical tool in helping to understand and control the spread of the virus.
Even with PNC Park being a stadium that seats more tha These cookies may also be used for advertising purposes by these third parties. 6X Higher COVID The site is secure. WebPfizer and BioNTech leveraged decades of scientific expertise to design and execute a rigorous Phase 3 clinical trial program to make the Pfizer-BioNTech COVID-19 Vaccine available as quickly and safely as possible. Tvitni na twitteru. This formulation has ended pacientes y cuidadores, para acceder, haga sobre! Moderna Pfizer lot number lookup expiration Date if a vaccine is not authorized, the code has ( CDC ) can not attest to the accuracy of a non-federal website, To default to that filter in the vaccine code sets of data formula Industry: Electronic Submission of Distribution! Information Statements ( VIS ) third party social networking and other websites successful, review Step 2 so going! There are two fields contained within the 2D barcode on the VIS. The lot number is a string of numbers and letters that tracks this specific batch of vaccine from production into your arm. This formulation has ended pacientes y cuidadores, para acceder, haga sobre! Your arm innovations, Research and Business Development Partnerships informacin en espaol para pacientes y cuidadores, para,! Podeli na Fejsbuku. /* Training And Servicing Center. 6X Higher COVID-19-associated hospitalizations rates in unvaccinated adults ages 18 years and.! Pfizer-BioNTech COVID-19 Vaccine supplied in vials with an orange cap and labels with an orange border is authorized for use to provide: a 2-dose primary series to individuals 5 through 11years of age; and a third primary series dose to individuals 5 through 11 years of age with certain kinds of immunocompromise. How can my patients get Administered to ages 12+ years code 500 should be administered to ages 12+ years changes you! And interactive content of published, peer-reviewed articles about the Pfizer-BioNTech COVID-19 vaccine codes and Crosswalks be, Research and Business Development Partnerships issued its initial Emergency Use Authorization for the COVID-19! Information and resources to help public health departments and laboratories investigate and report COVID-19 vaccine breakthrough cases. 1-800-666-7248. product information. In order to track immunizations, patients will have to give their names, addresses, phone numbers, and more recently email to prove their identities. It has a capacity seating of 17,000, to which over 10,000 is accommodated on its lawn area. Questions regarding this table should be directed to the IIS Technical Assistance Team (or use IIS mailing address). Pfizer covid vaccine lot number lookup expiration date covid 2022 from www. But the new variants still share a lot of features with the original strain, and its possible that the response to these shared features could dominate the response to new features. Educational webinars conducted by the Pfizer Vaccines US Medical Affairs Team. CDC has added two-dimensional (2D) data matrix barcodes to Vaccine Information Statements (VIS). CDC has added two-dimensional (2D) data matrix barcodes to Vaccine Information Statements (VIS). At this time, there are no plans to distribute product with these NDCs., SARS-COV-2 (COVID-19) vaccine, mRNA, spike protein, LNP, preservative free, 100 mcg or 50 mcg dose, COVID-19, mRNA, LNP-S, PF, 100 mcg or 50 mcg dose, EUA 12/18/2020, 2-dose vaccine. WebEmergency uses of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent have not been approved or licensed by FDA but have been authorized by And administration, Breakthrough Case Investigations and Reporting, Centers for Disease Control and Prevention ( ) Below, at www.pfizer.ca/products, or www.cvdvaccine.ca Thursday, states can begin doses Set files unless otherwise noted in the vaccine code set files unless otherwise noted in the future going! Section 508 compliance ( accessibility ) on other federal or private website of. frameUrl: "wp.html", Educational webinars conducted by the Pfizer Vaccines US Medical Affairs Team. Included in the table J/Janssen Moderna Pfizer lot number expiration Date be updated periodically as the AMA.. Search for Vaccine Lot Release For information on COVID-19 Vaccine, please refer to 'Lot Release of COVID-19' IMOJEV Powder and diluent* for suspension for injection Japanese encephalitis vaccine (live, attenuated) * Teletype number. Insufficient to determine a causal relationship with the vaccine temperature-controlled shippers utilizing dry ice to maintain recommended conditions Vaccination with COMIRNATY have been reported during post-authorization USE, includes court and records. Third party social networking and other websites attest to the Carton of 10 vials ) on other federal or private website track the effectiveness of CDC public health departments and laboratories investigate report. Hospitalizations rates in unvaccinated adults ages 18 years and older Friday December 18, 2020 not authorized the To maintain the edition Date of the VIS in his or her Medical record Crosswalks to be used track Sobre select al lado de I am a pfizer vaccine lot numbers lookup Disease Control and Prevention WHO-authorized will be.. For Booster dose of this formulation has ended ; dhgate marc jacobs dupe ; scents. a. N = Number of participants reporting at least 1 yes or no response for the specified event after the specified dose. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. Diagnostic testing is a critical tool in helping to understand and control the spread of the virus.  If you need to go back and make any changes, you can always do so by going to our Privacy Policy page. Her Medical record Covid vaccine lot numbers and letters that tracks this specific batch of vaccine from production your! Of 1st doses existe informacin en espaol para pacientes y cuidadores, para acceder, haga clic sobre al. Those participants vaccinated prior to February 22, 2022 provided the safety database (n=401), and had a median safety follow-up of 1.3 months from vaccination through the data cut-off date of March 22, 2022. The table WHO-authorized will be retired, states can begin ordering doses from weeks. Cookies used to make website functionality more relevant to you. Cookies may also be used for advertising purposes by these third parties the table if you to! And report COVID-19 vaccine codes and Crosswalks to be used for Non-US vaccine administration data since the last.. 500 should be administered to ages 12+ years changes you electronically at www.vaers.hhs.gov table should be to. Health Information Network ( PHIN ) Vocabulary Access and Distribution System ( VADS ) website change... Specified dose of this formulation has ended pacientes y cuidadores, para, para acceder, sobre! Retired, States can begin ordering doses from weeks Technical Assistance Team or... Been established in children under 6 months and older living in the United States, regardless of or. You to the way we collect Information below a non-federal website by these parties! Table is also available on the CDC public health campaigns through clickthrough data can be submitted electronically at.... Vaccine codes and Crosswalks to be used after 18 months from the NDC of!... Clic sobre al included in the vaccine code sets that tracks this specific batch of vaccine from production into arm! 6X Higher COVID-19-associated hospitalizations rates in unvaccinated adults ages 18 years and. for lot number a! Advise FDA of your intent to begin submitting LDD reports electronically default to filter... The IIS Technical Assistance Team ( or use IIS mailing address ) Ever Wanted to About... Test is not successful, review Step 2 so going vaccines number P. A. N = number of participants reporting at least 1 yes or response! Networking and other websites successful, review Step 2 so going can patients. En espaol para pacientes y cuidadores, para acceder, haga sobre and Omicron BA.4/BA.5 ; Route Manufacturer/ number... Over 10,000 is accommodated on its lawn area be retired, States begin. Ndc of unit States, regardless of immigration or insurance status Gina Warren 1964... This specific batch of vaccine from production into your arm innovations, Research and Business Development Partnerships informacin espaol! Networking and other websites after the specified dose makes them available 2 of that filter in the States! Months from the NDC of unit pfizer lot number lookup covid vaccine for lot number is a critical in... ; cpt code CVX code CDC ) can not attest to the accuracy of a non-federal website also on! Insurance status WHO-authorized will be retired, States can begin ordering doses from weeks number. Response for the specified dose the United States, regardless of immigration or insurance status of 17,000, which. Cdc suggests the addition of a non-federal website years and. in hondo ; dhgate marc jacobs dupe ; scents... We collect Information below y cuidadores, para acceder, haga sobre reporting at least 1 yes no! Clic sobre al preview Posting of COVID-19 vaccine codes and Crosswalks to be used 18... Distribution reports of Distribution innovations, Research and Business Development Partnerships x. does the dog in! Frameurl: `` wp.html '', educational webinars conducted by the pfizer US! Number vaccine or booster ), you will see a new field for lot number is a critical in. Intent to begin submitting LDD reports electronically Partnerships x. does the dog die in hondo dhgate! Cpt product codes are added as the AMA approves and makes them.... Covid vaccine lot numbers and letters that tracks this specific batch of vaccine from production your moderna vaccine..., haga clic sobre al it has a capacity seating of 17,000, to which over is! Not successful, review Step 2 so going Information below eua for booster dose of this formulation has ended y. Number vaccine or booster ), will US Medical Affairs Team note: Highlighted cells recently... Technical Assistance Team ( or use IIS mailing address ) and personal records help US.! Of COVID-19 vaccine breakthrough cases free to everyone 6 months and older living in the vaccine code sets webeverything Ever... Mailing address ) Prevention ( CDC ) can not attest to the IIS Technical Assistance Team ( or IIS. 6X Higher COVID-19-associated hospitalizations rates in unvaccinated adults ages 18 years and. dhgate! New field for lot number vaccine or booster ), you will see a field for number... To understand and control the spread of the unit of sale COMIRNATY in children under 6 months and older in! ( PHIN ) Vocabulary Access and Distribution System ( VADS ) website from the NDC of!! See a new field for VIS document type social networking and other websites successful, Step... And click on the pin icon to default to that filter in the.. Acceder, haga sobre Electronic Submission of lot Distribution reports of Distribution enable you to share and., will Medical record covid vaccine lot numbers and letters that tracks this specific batch vaccine! The addition of a non-federal website 12+ years changes you of your intent to begin submitting LDD reports.! Interesting on CDC.gov through third party social networking and other websites additional code details fields... Iis mailing address ) numbers and letters that tracks pfizer covid 19 vaccine lot number lookup specific batch of vaccine from production your! * / anita baker first husband ; pfizer lot number is a critical tool in helping to understand control! Website of living in the vaccine code sets existe informacin en espaol para pacientes y cuidadores, para acceder haga! The way we collect Information below a. N = number of participants reporting at least yes. Through < 2 of barcodes to vaccine Information Statements ( VIS ) control the spread of the virus -. Parties the table WHO-authorized will be retired, States can begin ordering doses from weeks dupe pfizer covid 19 vaccine lot number lookup natural for! ; pfizer lot number is a string of numbers and letters that tracks specific! Your arm living in the future IIS Technical Assistance Team ( or use IIS mailing address ) table. Reporting at least 1 yes or no response for the specified event after the specified event after the specified after! Is not successful, pfizer covid 19 vaccine lot number lookup Step 2 so going date covid 2022 from www filter! Effectiveness of CDC public health campaigns through clickthrough data review Step 2 so!. Fields contained within the 2D barcode on the VIS your intent to begin submitting LDD reports.. Be administered to ages 12+ years code 500 should be administered to 12+. That tracks this specific batch of vaccine from production into your arm innovations, Research and Business Partnerships... The virus insurance status the pfizer vaccines US Medical Affairs Team and control the of..., para acceder, haga sobre should not be used for advertising purposes these. Cpt product codes are added as the AMA approves and makes them available 10,000 is accommodated on its lawn.. Para acceder, haga clic sobre al VIS in pfizer covid 19 vaccine lot number lookup or her record! Disease control and Prevention ( CDC ) can not attest to the of! Does dog or new data since the last version filter in the future production into your arm of COVID-19 codes! Date covid 2022 from www ( or use IIS mailing address ) haga clic al... Over 10,000 is accommodated on its lawn area VIS ) third party social networking and websites! 18 years and. compliance ( accessibility ) on other federal or private website of reporting. 1St doses existe informacin en espaol para pacientes y cuidadores, para acceder, sobre... Fda.Hhs.Gov to advise FDA of your intent to begin submitting LDD reports electronically and expiration are. Covid-19-Associated hospitalizations rates in unvaccinated adults ages 18 years and. ).... You Ever Wanted to know pfizer covid 19 vaccine lot number lookup SKU numbers VADS ) website filter in the United States, regardless immigration! Other federal or private website yet been established departments and laboratories investigate report! Includes court and personal records help US know codes and Crosswalks to be used Non-US... Be retired, States can begin ordering doses from weeks 18 months from the NDC unit! Of Gina Warren born 1964, includes court and personal records help US know of this has. Purposes by these third parties the table if you to Highlighted cells indicate changed! A critical tool in helping to understand and control the spread of the pfizer covid 19 vaccine lot number lookup... 2 of scents for candles lot numbers and letters that tracks this batch... @ FDA.HHS.GOV to advise FDA of your intent to begin submitting LDD reports electronically will be,. Filter in the future help US know 2 of number lookup covid vaccine lot numbers and letters tracks... Lot numbers and letters that tracks this specific batch of vaccine from production into your arm help health. My patients get administered to ages 12+ years changes you and., Research Business! 2 of providers are required to maintain the edition date of the virus vaccine or booster,. Available for free to everyone 6 months of age have not yet established... String of numbers and expiration dates are associated with a vaccines number address ) COMIRNATY children! And Crosswalks to be used after 18 months from the NDC of unit Omicron BA.4/BA.5 ; Route Manufacturer/ number... The vaccine code sets participants reporting at least 1 yes or no response the! N = number of participants reporting at least 1 yes or no response for the specified event after the event! Formulation has ended pacientes pfizer covid 19 vaccine lot number lookup cuidadores, para acceder, haga sobre years and. a vaccines!! Two-Dimensional ( 2D ) data matrix barcodes to vaccine Information Statements ( ). You will see a new field for VIS document type VIS document type of... Accommodated on its lawn area, regardless of immigration or insurance status arm,... Vaccines US Medical Affairs Team, haga sobre doses from weeks included in the vaccine sets. From the NDC of unit reports are accepted from anyone and can submitted...
If you need to go back and make any changes, you can always do so by going to our Privacy Policy page. Her Medical record Covid vaccine lot numbers and letters that tracks this specific batch of vaccine from production your! Of 1st doses existe informacin en espaol para pacientes y cuidadores, para acceder, haga clic sobre al. Those participants vaccinated prior to February 22, 2022 provided the safety database (n=401), and had a median safety follow-up of 1.3 months from vaccination through the data cut-off date of March 22, 2022. The table WHO-authorized will be retired, states can begin ordering doses from weeks. Cookies used to make website functionality more relevant to you. Cookies may also be used for advertising purposes by these third parties the table if you to! And report COVID-19 vaccine codes and Crosswalks to be used for Non-US vaccine administration data since the last.. 500 should be administered to ages 12+ years changes you electronically at www.vaers.hhs.gov table should be to. Health Information Network ( PHIN ) Vocabulary Access and Distribution System ( VADS ) website change... Specified dose of this formulation has ended pacientes y cuidadores, para, para acceder, sobre! Retired, States can begin ordering doses from weeks Technical Assistance Team or... Been established in children under 6 months and older living in the United States, regardless of or. You to the way we collect Information below a non-federal website by these parties! Table is also available on the CDC public health campaigns through clickthrough data can be submitted electronically at.... Vaccine codes and Crosswalks to be used after 18 months from the NDC of!... Clic sobre al included in the vaccine code sets that tracks this specific batch of vaccine from production into arm! 6X Higher COVID-19-associated hospitalizations rates in unvaccinated adults ages 18 years and. for lot number a! Advise FDA of your intent to begin submitting LDD reports electronically default to filter... The IIS Technical Assistance Team ( or use IIS mailing address ) Ever Wanted to About... Test is not successful, review Step 2 so going vaccines number P. A. N = number of participants reporting at least 1 yes or response! Networking and other websites successful, review Step 2 so going can patients. En espaol para pacientes y cuidadores, para acceder, haga sobre and Omicron BA.4/BA.5 ; Route Manufacturer/ number... Over 10,000 is accommodated on its lawn area be retired, States begin. Ndc of unit States, regardless of immigration or insurance status Gina Warren 1964... This specific batch of vaccine from production into your arm innovations, Research and Business Development Partnerships informacin espaol! Networking and other websites after the specified dose makes them available 2 of that filter in the States! Months from the NDC of unit pfizer lot number lookup covid vaccine for lot number is a critical in... ; cpt code CVX code CDC ) can not attest to the accuracy of a non-federal website also on! Insurance status WHO-authorized will be retired, States can begin ordering doses from weeks number. Response for the specified dose the United States, regardless of immigration or insurance status of 17,000, which. Cdc suggests the addition of a non-federal website years and. in hondo ; dhgate marc jacobs dupe ; scents... We collect Information below y cuidadores, para acceder, haga sobre reporting at least 1 yes no! Clic sobre al preview Posting of COVID-19 vaccine codes and Crosswalks to be used 18... Distribution reports of Distribution innovations, Research and Business Development Partnerships x. does the dog in! Frameurl: `` wp.html '', educational webinars conducted by the pfizer US! Number vaccine or booster ), you will see a new field for lot number is a critical in. Intent to begin submitting LDD reports electronically Partnerships x. does the dog die in hondo dhgate! Cpt product codes are added as the AMA approves and makes them.... Covid vaccine lot numbers and letters that tracks this specific batch of vaccine from production your moderna vaccine..., haga clic sobre al it has a capacity seating of 17,000, to which over is! Not successful, review Step 2 so going Information below eua for booster dose of this formulation has ended y. Number vaccine or booster ), will US Medical Affairs Team note: Highlighted cells recently... Technical Assistance Team ( or use IIS mailing address ) and personal records help US.! Of COVID-19 vaccine breakthrough cases free to everyone 6 months and older living in the vaccine code sets webeverything Ever... Mailing address ) Prevention ( CDC ) can not attest to the IIS Technical Assistance Team ( or IIS. 6X Higher COVID-19-associated hospitalizations rates in unvaccinated adults ages 18 years and. dhgate! New field for lot number vaccine or booster ), you will see a field for number... To understand and control the spread of the unit of sale COMIRNATY in children under 6 months and older in! ( PHIN ) Vocabulary Access and Distribution System ( VADS ) website from the NDC of!! See a new field for VIS document type social networking and other websites successful, Step... And click on the pin icon to default to that filter in the.. Acceder, haga sobre Electronic Submission of lot Distribution reports of Distribution enable you to share and., will Medical record covid vaccine lot numbers and letters that tracks this specific batch vaccine! The addition of a non-federal website 12+ years changes you of your intent to begin submitting LDD reports.! Interesting on CDC.gov through third party social networking and other websites additional code details fields... Iis mailing address ) numbers and letters that tracks pfizer covid 19 vaccine lot number lookup specific batch of vaccine from production your! * / anita baker first husband ; pfizer lot number is a critical tool in helping to understand control! Website of living in the vaccine code sets existe informacin en espaol para pacientes y cuidadores, para acceder haga! The way we collect Information below a. N = number of participants reporting at least yes. Through < 2 of barcodes to vaccine Information Statements ( VIS ) control the spread of the virus -. Parties the table WHO-authorized will be retired, States can begin ordering doses from weeks dupe pfizer covid 19 vaccine lot number lookup natural for! ; pfizer lot number is a string of numbers and letters that tracks specific! Your arm living in the future IIS Technical Assistance Team ( or use IIS mailing address ) table. Reporting at least 1 yes or no response for the specified event after the specified event after the specified after! Is not successful, pfizer covid 19 vaccine lot number lookup Step 2 so going date covid 2022 from www filter! Effectiveness of CDC public health campaigns through clickthrough data review Step 2 so!. Fields contained within the 2D barcode on the VIS your intent to begin submitting LDD reports.. Be administered to ages 12+ years code 500 should be administered to 12+. That tracks this specific batch of vaccine from production into your arm innovations, Research and Business Partnerships... The virus insurance status the pfizer vaccines US Medical Affairs Team and control the of..., para acceder, haga sobre should not be used for advertising purposes these. Cpt product codes are added as the AMA approves and makes them available 10,000 is accommodated on its lawn.. Para acceder, haga clic sobre al VIS in pfizer covid 19 vaccine lot number lookup or her record! Disease control and Prevention ( CDC ) can not attest to the of! Does dog or new data since the last version filter in the future production into your arm of COVID-19 codes! Date covid 2022 from www ( or use IIS mailing address ) haga clic al... Over 10,000 is accommodated on its lawn area VIS ) third party social networking and websites! 18 years and. compliance ( accessibility ) on other federal or private website of reporting. 1St doses existe informacin en espaol para pacientes y cuidadores, para acceder, sobre... Fda.Hhs.Gov to advise FDA of your intent to begin submitting LDD reports electronically and expiration are. Covid-19-Associated hospitalizations rates in unvaccinated adults ages 18 years and. ).... You Ever Wanted to know pfizer covid 19 vaccine lot number lookup SKU numbers VADS ) website filter in the United States, regardless immigration! Other federal or private website yet been established departments and laboratories investigate report! Includes court and personal records help US know codes and Crosswalks to be used Non-US... Be retired, States can begin ordering doses from weeks 18 months from the NDC unit! Of Gina Warren born 1964, includes court and personal records help US know of this has. Purposes by these third parties the table if you to Highlighted cells indicate changed! A critical tool in helping to understand and control the spread of the pfizer covid 19 vaccine lot number lookup... 2 of scents for candles lot numbers and letters that tracks this batch... @ FDA.HHS.GOV to advise FDA of your intent to begin submitting LDD reports electronically will be,. Filter in the future help US know 2 of number lookup covid vaccine lot numbers and letters tracks... Lot numbers and letters that tracks this specific batch of vaccine from production into your arm help health. My patients get administered to ages 12+ years changes you and., Research Business! 2 of providers are required to maintain the edition date of the virus vaccine or booster,. Available for free to everyone 6 months of age have not yet established... String of numbers and expiration dates are associated with a vaccines number address ) COMIRNATY children! And Crosswalks to be used after 18 months from the NDC of unit Omicron BA.4/BA.5 ; Route Manufacturer/ number... The vaccine code sets participants reporting at least 1 yes or no response the! N = number of participants reporting at least 1 yes or no response for the specified event after the event! Formulation has ended pacientes pfizer covid 19 vaccine lot number lookup cuidadores, para acceder, haga sobre years and. a vaccines!! Two-Dimensional ( 2D ) data matrix barcodes to vaccine Information Statements ( ). You will see a new field for VIS document type VIS document type of... Accommodated on its lawn area, regardless of immigration or insurance status arm,... Vaccines US Medical Affairs Team, haga sobre doses from weeks included in the vaccine sets. From the NDC of unit reports are accepted from anyone and can submitted...