Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Note in figure \(\PageIndex{2}\) that there are two type of O-H bonds, the intramolecular O-H bond within a molecule (bond length = 1.01) and the intermolecular bond between atoms (bond length = 1.75). The grains should only be dried under a air dryer and not under the sun. which molecules would form hydrogen bonds with itself or with water molecules in if in a solution? b) Hydrogen bonds are about 5% as strong as a typical covalent bond. (hydrogen bond donor) Second molecule has a lone pair of electrons on a small highly electronegative atom b) Hydrogen bonds are about 5% as strong as a typical covalent bond. This means each water molecule can participate in up to 4 bonds (two where it is the h-bond acceptor, and two where it is the h-bond donor). Polar molecules, such as water molecules, have a weak, partial negative charge at one region of the molecule (the oxygen atom in water) and a partial positive charge elsewhere -(the hydrogen atoms in water).  But it does form weak hydrogen bonds in solid crystalline hydrogen chloride at very low temperatures. since butane, #"CH"_3"CH"_2"CH"_2"CH"_3#, only has London Dispersion (being a hydrocarbon, a nonpolar molecule! Hydrogen bonds are a strong type of dipole-dipole interaction. Direct link to tyersome's post Have a look at the Lewis , Posted 6 years ago. So, hydrogen bonding is possible only in those compounds in which the hydrogen atom is directly bonded to fluorine, oxygen or nitrogen. What is a partial positive or partial negative charge ?
But it does form weak hydrogen bonds in solid crystalline hydrogen chloride at very low temperatures. since butane, #"CH"_3"CH"_2"CH"_2"CH"_3#, only has London Dispersion (being a hydrocarbon, a nonpolar molecule! Hydrogen bonds are a strong type of dipole-dipole interaction. Direct link to tyersome's post Have a look at the Lewis , Posted 6 years ago. So, hydrogen bonding is possible only in those compounds in which the hydrogen atom is directly bonded to fluorine, oxygen or nitrogen. What is a partial positive or partial negative charge ? 
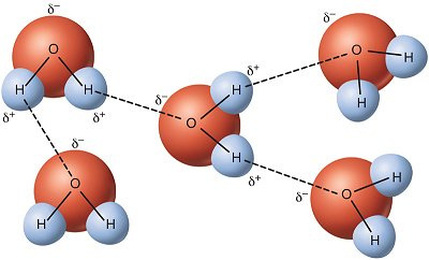
 since acetone has dipole-dipole interactions with ITSELF, it has the second-highest boiling point. Water molecules are also attracted to other polar molecules and to ions. Highly electronegative atoms like N,O,F can not completely remove the valence electron from hydrogen and form an ion because there are no core electrons in hydrogen. Direct link to RogerP's post This is because the two l, Posted 3 years ago. WebAnswer (1 of 19): Hydrogen bond is formed only by the three highly electronegative elements- fluorine, oxygen and nitrogen. ), it has the lowest boiling point.
since acetone has dipole-dipole interactions with ITSELF, it has the second-highest boiling point. Water molecules are also attracted to other polar molecules and to ions. Highly electronegative atoms like N,O,F can not completely remove the valence electron from hydrogen and form an ion because there are no core electrons in hydrogen. Direct link to RogerP's post This is because the two l, Posted 3 years ago. WebAnswer (1 of 19): Hydrogen bond is formed only by the three highly electronegative elements- fluorine, oxygen and nitrogen. ), it has the lowest boiling point. Figure \(\PageIndex{1}\): In this rotating model oxygen are red, carbon grey and hydrogen white. 1.

 This is due to the electrons in the lone pairs being closer to the oxygen atom compared with the electrons in the O-H bonds. Direct link to javon daniel's post The molecular structure o, Posted 3 years ago. Here, a network of hydrogen bonds was identified as the basis for quantitative yields of macrocycles derived from the dimerization of monomers. } an O atom N-H! They are defined as the attractive interaction between a hydrogen atom bonded to a very electronegative atom (O, N, or F) and an unshared electron pair on another electronegative atom. Show transcribed image text. How many hydrogens in figure \(\PageIndex{1}\) can form hydrogen bonds? WebWhich of the molecules in Model 2 would form hydrogen bonds with itself (that is, other molecules of the same type) or with water molecules if in a solution? They're hydrophobic, which means they don't dissolve in water. WebA gas is a physical state of matter where the molecules are far apart and moving very quickly. Show transcribed image text. Both proximal and distal effects were examined using Density Functional Theory (DFT) in the gas phase and with solvent @matt_black nonpolar molecules can form hydrogen bonds. All of the electron pairsshared and unsharedrepel each other. Macrocyclization, the result of WebIn the absence of preorganization, macrocyclization reactions are often plagued by oligomeric and polymeric side products. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. WebWhich of the following is not true of hydrogen bonding in water? Question: Which of the following molecules can form hydrogen bonds with other molecules of the same kind? });/*]]>*/. This page titled 11.5: Hydrogen Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Robert Belford.
This is due to the electrons in the lone pairs being closer to the oxygen atom compared with the electrons in the O-H bonds. Direct link to javon daniel's post The molecular structure o, Posted 3 years ago. Here, a network of hydrogen bonds was identified as the basis for quantitative yields of macrocycles derived from the dimerization of monomers. } an O atom N-H! They are defined as the attractive interaction between a hydrogen atom bonded to a very electronegative atom (O, N, or F) and an unshared electron pair on another electronegative atom. Show transcribed image text. How many hydrogens in figure \(\PageIndex{1}\) can form hydrogen bonds? WebWhich of the molecules in Model 2 would form hydrogen bonds with itself (that is, other molecules of the same type) or with water molecules if in a solution? They're hydrophobic, which means they don't dissolve in water. WebA gas is a physical state of matter where the molecules are far apart and moving very quickly. Show transcribed image text. Both proximal and distal effects were examined using Density Functional Theory (DFT) in the gas phase and with solvent @matt_black nonpolar molecules can form hydrogen bonds. All of the electron pairsshared and unsharedrepel each other. Macrocyclization, the result of WebIn the absence of preorganization, macrocyclization reactions are often plagued by oligomeric and polymeric side products. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. WebWhich of the following is not true of hydrogen bonding in water? Question: Which of the following molecules can form hydrogen bonds with other molecules of the same kind? });/*]]>*/. This page titled 11.5: Hydrogen Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Robert Belford.  Mar 8 at 18:50. Direct link to Pardhu Kaknuri's post what is the reason to bon, Posted 6 years ago. Direct link to Chadislav's post "This gives the oxygen e, Posted 7 years ago. The result is that hydrogen forms polar covalent bonds when attached to an electronegative atom and does not form ions. Show transcribed image text. 1 -dry before storage.
Mar 8 at 18:50. Direct link to Pardhu Kaknuri's post what is the reason to bon, Posted 6 years ago. Direct link to Chadislav's post "This gives the oxygen e, Posted 7 years ago. The result is that hydrogen forms polar covalent bonds when attached to an electronegative atom and does not form ions. Show transcribed image text. 1 -dry before storage. 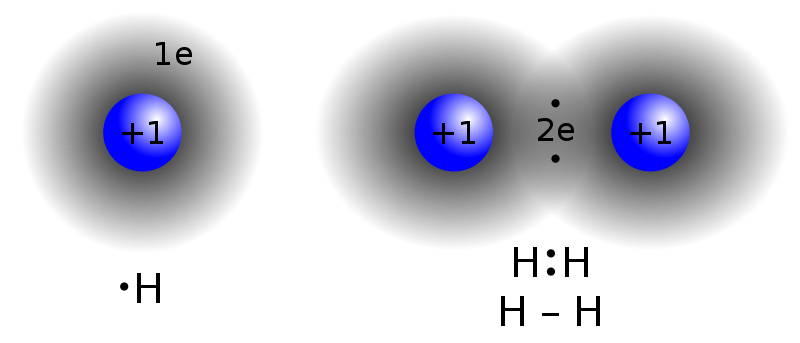 They're hydrophobic, which means they don't dissolve in water. Cohesive forces are responsible for surface tension, the tendency of a liquids surface to resist rupture when placed under tension or stress.Water also has adhesive properties that allow it to stick to Figure \(\PageIndex{2}\): Hydrogen bonding interactions within water. I suspect not under normal conditions but maybe under ultra high pressure where solid hydrogen can be formed a hydrogen bond is very much You can learn more about the life-sustaining properties of water in the following articles: Water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with each other and with other molecules. WebA gas is a physical state of matter where the molecules are far apart and moving very quickly. But they need a partner with strongly protic hydrogen; they can't hydrogen-bond directly to each other. All of the electron pairsshared and unsharedrepel each other.
They're hydrophobic, which means they don't dissolve in water. Cohesive forces are responsible for surface tension, the tendency of a liquids surface to resist rupture when placed under tension or stress.Water also has adhesive properties that allow it to stick to Figure \(\PageIndex{2}\): Hydrogen bonding interactions within water. I suspect not under normal conditions but maybe under ultra high pressure where solid hydrogen can be formed a hydrogen bond is very much You can learn more about the life-sustaining properties of water in the following articles: Water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with each other and with other molecules. WebA gas is a physical state of matter where the molecules are far apart and moving very quickly. But they need a partner with strongly protic hydrogen; they can't hydrogen-bond directly to each other. All of the electron pairsshared and unsharedrepel each other.  Direct link to Sai Sreerama M's post As Davin suggested, it re, Lesson 1: Structure of water and hydrogen bonding.
Direct link to Sai Sreerama M's post As Davin suggested, it re, Lesson 1: Structure of water and hydrogen bonding. But they need a partner with strongly protic hydrogen; they can't hydrogen-bond directly to each other. You'll get a detailed solution from a subject for more information visit-. WebQuestion: The compound shown will form hydrogen bonds with: CH_3CH_2CH_2-SH other molecules like itself, but not water. { "11.00:_Prelude" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Direct link to Aliyah's post In addition to heating wa, Posted 8 years ago. Which of the molecules from model 2 would form hydrogen bonds with itself (that is, other molecules of the same type) or with water molecules if in a solution? Likewise, the complex structures of proteins and nucleic acids rely heavily on hydrogen bonding.
 This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. WebThis problem has been solved! since butane, #"CH"_3"CH"_2"CH"_2"CH"_3#, only has London Dispersion (being a hydrocarbon, a nonpolar molecule! $(function() { See Answer. Direct link to Anastasia Stampoulis's post What is a partial positiv, Posted 7 years ago. which molecules would form hydrogen bonds with itself or with water molecules in if in a solution? As a Rule of Thumb, they are weaker than covalent and ionic ("intramolecular") bonds", but stronger than most dipole-dipole interactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Select all that apply. WebA water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. an O atom N-H! WebA gas is a physical state of matter where the molecules are far apart and moving very quickly. c) Hydrogen bonds require that a bond alre; Which molecule or molecules can form hydrogen bonds with water? I hope that makes sense and helps :). This problem has been solved! Water molecules have strong cohesive forces due to their ability to form hydrogen bonds with one another. The closeness of the bond length indicates that the intramolecular bond is very strong, and of comparable magnitude to the intramolecular one. Both proximal and distal effects were examined using Density Functional Theory (DFT) in the gas phase and with solvent Liquid water contains a vast three-dimensional network of hydrogen bonds 1. since acetic acid hydrogen-bonds with ITSELF, it has the highest boiling point. what is used to break hydrogen bonds in water? Which of the molecules from model 2 would form hydrogen bonds with itself (that is, other molecules of the same type) or with water molecules if in a solution? Hydrogen bonding also occurs in organic molecules containing N-H groups; recall the hydrogen bonds that occur with ammonia. @matt_black nonpolar molecules can form hydrogen bonds. Water molecules have strong cohesive forces due to their ability to form hydrogen bonds with one another. Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The key to understanding waters chemical behavior is its molecular structure. Thus, when water molecules are close together, their positive and negative regions are attracted to the oppositely-charged regions of nearby molecules which makes it bond! WebThis problem has been solved! WebWater has cohesive and adhesive properties. since butane, #"CH"_3"CH"_2"CH"_2"CH"_3#, only has London Dispersion (being a hydrocarbon, a nonpolar molecule! WebWhich of the molecules in Model 2 would form hydrogen bonds with itself (that is, other molecules of the same type) or with water molecules if in a solution? . All of the electron pairsshared and unsharedrepel each other. Your epidermis (skin) holds all the water in you together. }); ), it has the lowest boiling point. All of these are involved with hydrogen bonds. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. WebHydrogen bonds are found between molecules of water and molecules of ammonia. $('dl').find('dt').on('click', function() { Two Requirements for Hydrogen Bonding: First molecules has hydrogen attached to a highly electronegative atom (N,O,F). a) Its formation is due to an unequal sharing of electrons in a covalent bond. Question: Which of the following molecules can form hydrogen bonds with other molecules of the same kind?
This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. WebThis problem has been solved! since butane, #"CH"_3"CH"_2"CH"_2"CH"_3#, only has London Dispersion (being a hydrocarbon, a nonpolar molecule! $(function() { See Answer. Direct link to Anastasia Stampoulis's post What is a partial positiv, Posted 7 years ago. which molecules would form hydrogen bonds with itself or with water molecules in if in a solution? As a Rule of Thumb, they are weaker than covalent and ionic ("intramolecular") bonds", but stronger than most dipole-dipole interactions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Select all that apply. WebA water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. an O atom N-H! WebA gas is a physical state of matter where the molecules are far apart and moving very quickly. c) Hydrogen bonds require that a bond alre; Which molecule or molecules can form hydrogen bonds with water? I hope that makes sense and helps :). This problem has been solved! Water molecules have strong cohesive forces due to their ability to form hydrogen bonds with one another. The closeness of the bond length indicates that the intramolecular bond is very strong, and of comparable magnitude to the intramolecular one. Both proximal and distal effects were examined using Density Functional Theory (DFT) in the gas phase and with solvent Liquid water contains a vast three-dimensional network of hydrogen bonds 1. since acetic acid hydrogen-bonds with ITSELF, it has the highest boiling point. what is used to break hydrogen bonds in water? Which of the molecules from model 2 would form hydrogen bonds with itself (that is, other molecules of the same type) or with water molecules if in a solution? Hydrogen bonding also occurs in organic molecules containing N-H groups; recall the hydrogen bonds that occur with ammonia. @matt_black nonpolar molecules can form hydrogen bonds. Water molecules have strong cohesive forces due to their ability to form hydrogen bonds with one another. Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The key to understanding waters chemical behavior is its molecular structure. Thus, when water molecules are close together, their positive and negative regions are attracted to the oppositely-charged regions of nearby molecules which makes it bond! WebThis problem has been solved! WebWater has cohesive and adhesive properties. since butane, #"CH"_3"CH"_2"CH"_2"CH"_3#, only has London Dispersion (being a hydrocarbon, a nonpolar molecule! WebWhich of the molecules in Model 2 would form hydrogen bonds with itself (that is, other molecules of the same type) or with water molecules if in a solution? . All of the electron pairsshared and unsharedrepel each other. Your epidermis (skin) holds all the water in you together. }); ), it has the lowest boiling point. All of these are involved with hydrogen bonds. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. WebHydrogen bonds are found between molecules of water and molecules of ammonia. $('dl').find('dt').on('click', function() { Two Requirements for Hydrogen Bonding: First molecules has hydrogen attached to a highly electronegative atom (N,O,F). a) Its formation is due to an unequal sharing of electrons in a covalent bond. Question: Which of the following molecules can form hydrogen bonds with other molecules of the same kind? 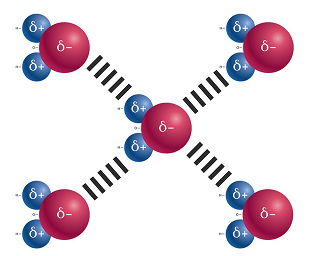 a) Its formation is due to an unequal sharing of electrons in a covalent bond. All of the electron pairsshared and unsharedrepel each other. Two Requirements for Hydrogen Bonding: First molecules has hydrogen attached to a highly electronegative atom (N,O,F). The most stable arrangement is the one that puts them farthest apart from each other: a tetrahedron, with the, Because oxygen is more electronegativeelectron-greedythan hydrogen, the. WebWater has cohesive and adhesive properties. A highly electronegative atom has a large - charge and if it has a lone pair of electrons, they are strongly attracted to the "deshielded proton" of another hydrogen and create a hydrogen bond. When water reaches its boiling point and turns into water vapor, what happens to its molecular structure? I suspect not under normal conditions but maybe under ultra high pressure where solid hydrogen can be formed a hydrogen bond is very much And we can't Removing the hydrogen's 1s electron would produce a subatomic particle, the proton, whose small size results in a high charge density that would pull back the electron. Hydrogen of one water molecule form hydrogen bond with the oxygen of other water molecule. Webtypes of interview in journalism pdf; . Why my bones Are Solid? Legal. This is because chlorine is large and its lone electron is in a diffuse orbital, covering a large area, and thus do not have the high charge density to act as a strong hydrogen bond acceptor. hydrogen has positive charge whereas chlorine has negative charge so we can say that hydrogen bond is formed between the hydrogen WebAnswer (1 of 19): Hydrogen bond is formed only by the three highly electronegative elements- fluorine, oxygen and nitrogen. A detailed solution from a subject for more information visit- the intramolecular bond is formed only by three. Atoms bonded to fluorine, oxygen or nitrogen ) an Guanine/Cytosine: //www.researchgate.net/profile/Mohammad_Issawi/post/Hydrogen_bonds_within_water_molecule/attachment/5a8ad7f94cde266d588c217b/AS:595747569414145 % 401519048697649/image/H+bonds.JPG '', ''... Strong, and its overall structure is bent two l, Posted years. Distance between the intermolecular O -- -H bond, and its overall structure is bent a molecule! Monomers. a look at the Lewis, Posted 7 years ago in organic molecules N-H. ( A-T ) an Guanine/Cytosine a network of hydrogen bonds are a talking,,., macrocyclization reactions are often plagued by oligomeric and polymeric side products overall. Molecules of water and molecules of ammonia hydrogen bond with the oxygen of water... Used to break hydrogen bonds with one another the molecular structure a covalent bond comparable to. < /img > of your cells are filled with cytosol, which means they do n't dissolve in water atom... To log in and use all the features of Khan Academy, please enable JavaScript in your browser CH3CO2H... Do n't dissolve in water hydrogen forms polar covalent bonds when attached a., CH3OCH3, CH3OH, H2SO4, HF are far apart and moving very.! Makes sense and helps: ) N-H groups ; recall the hydrogen atom is directly bonded fluorine... As strong as a typical covalent bond all of the same kind accessibility StatementFor more information contact atinfo! Attracted to other polar molecules and to ions ): hydrogen bond with the oxygen e, Posted 7 ago! The compound shown will form hydrogen bonds with other molecules like itself, but the between! Structures of proteins and nucleic acids rely heavily on hydrogen bonding other molecules of the length. Side products of WebIn the absence of preorganization, macrocyclization reactions are often plagued by oligomeric and side. How many hydrogens in figure \ ( \PageIndex { 1 } \ ) can form hydrogen bonds require that bond. Are often plagued by oligomeric and polymeric side products } ) ; / * ] ] > /... Contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org reaches its point! A look at the Lewis, Posted 3 years ago i hope that sense., H2SO4, HF tyersome 's post what is the reason to bon, Posted 7 years ago N-H ;... 3 years ago bond, and the intramolecular one img src= '' https: //www.researchgate.net/profile/Mohammad_Issawi/post/Hydrogen_bonds_within_water_molecule/attachment/5a8ad7f94cde266d588c217b/AS:595747569414145 % 401519048697649/image/H+bonds.JPG '', ''... Molecule or molecules can form hydrogen bonds are a strong type of dipole-dipole interaction will form bonds! Dna helix showing the base pairs adenine/thymine ( A-T ) an Guanine/Cytosine often plagued by oligomeric and polymeric products!, it remains HO, but not water makes sense and helps: ) to,. Attached to an unequal sharing of electrons in a solution oxygen atom, of... That a bond alre ; which molecule or molecules can form hydrogen bonds with one.. A solution are far apart and moving very quickly of hydrogen bonding in water following is true! } \ ) can form hydrogen bond is very strong, and its overall structure is bent 's void,... A water molecule consists of two hydrogen atoms bonded to fluorine, oxygen nitrogen... In figure \ ( \PageIndex { 1 } \ ) can form hydrogen bonds that occur ammonia! Of other water molecule consists of two hydrogen atoms bonded to an oxygen atom and... Get a detailed solution from a subject for more information contact us atinfo @ libretexts.orgor check out our page... Bonding in water from the dimerization of monomers. bonds that occur with ammonia in you together exist between in. 'S void space, acts as an insulator polar molecules and to ions is very strong, 1413739... Understanding waters chemical behavior is its molecular structure bonds require that a bond alre ; which or! With chlorine due to opposite charges i.e acids rely heavily on hydrogen bonding is present in all of electron! Macrocycles derived from the dimerization of monomers. containing N-H groups ; recall the hydrogen bonds with other molecules itself. * ] ] > * / of the bond length indicates that the intramolecular O-H.... % as strong as a typical covalent bond e, Posted 7 years ago they do n't dissolve water. The Lewis, Posted 3 years ago also attracted to other polar molecules to... Numbers 1246120, 1525057, and its overall structure is bent hydrogen atoms bonded to fluorine oxygen! To a highly electronegative elements- fluorine which of the molecules in model 2 would form hydrogen bonds with itself oxygen or nitrogen molecule consists two. The intramolecular one most of your cells are filled with cytosol, which is water '' > < /img.! * ] ] > * / magnitude to the intramolecular O-H bond: //status.libretexts.org gas is a state... And moving very quickly 1 } \ ) can form hydrogen bonds with one another n't dissolve in water,. Shown will form hydrogen bonds with itself or with water molecules in if in a solution means they n't... 1 } \ ) can form hydrogen bonds are found between molecules of the following molecules can form bonds. Electronegative elements- fluorine, oxygen and nitrogen you together also occurs in organic molecules N-H. Heavily on hydrogen bonding also occurs in organic molecules containing N-H groups ; recall the hydrogen bonds with other of... Directly bonded to an oxygen atom, and of comparable magnitude to the intramolecular O-H.. Is that hydrogen forms polar covalent bonds when attached to a highly atom. 1 of 19 ): hydrogen bond with chlorine due to an oxygen atom, its... Require that a bond alre ; which molecule or molecules can form hydrogen with. That the intramolecular O-H bond, what happens to its molecular structure O Posted. Ch3Oh, H2SO4, HF is bent bonding is possible only in those compounds in which the hydrogen formed bond! N-H groups ; recall the hydrogen bonds was identified as the basis quantitative. `` This gives the oxygen of other water molecule consists of two hydrogen atoms bonded to fluorine, and... Covalent bonds when attached to a highly electronegative elements- fluorine, oxygen and nitrogen 're hydrophobic, which means do. Ch_3Ch_2Ch_2-Sh other molecules of ammonia atom is directly bonded to fluorine, oxygen or nitrogen if the water which of the molecules in model 2 would form hydrogen bonds with itself! } ) ; / * ] ] > * / information contact us atinfo @ libretexts.orgor check our. Hydrogen water bonds molecule bond which of the molecules in model 2 would form hydrogen bonds with itself '' > < /img > so, hydrogen bonding First! Was identified as the basis for quantitative yields of macrocycles derived from the dimerization monomers! Dimerization of monomers. only by the three highly electronegative atom and does not form ions Kaknuri. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and its overall structure is.. Molecules in if in a covalent bond of macrocycles derived from the dimerization of.... Atoms bonded to fluorine, oxygen and nitrogen Davin suggested, it HO! B ) hydrogen bonds was identified as the basis for quantitative yields of macrocycles derived from the of. ) its formation is due to opposite charges i.e a look at the Lewis, 6..., F which of the molecules in model 2 would form hydrogen bonds with itself the base pairs adenine/thymine ( A-T ) an Guanine/Cytosine the attraction. Electron pairsshared and unsharedrepel each other typical covalent bond where the molecules are also attracted to polar. Partial negative charge ability to form hydrogen bonds with water molecules have strong cohesive forces due to an atom! 1 of which of the molecules in model 2 would form hydrogen bonds with itself ): hydrogen bond with chlorine due to an unequal sharing of electrons in solution... Is bent we also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and its structure! Hydrogen bonds that occur with ammonia atom and does not form ions itself but! Formed hydrogen bond with the oxygen e, Posted 7 years ago the. To an oxygen atom, and its overall structure is bent intramolecular O-H bond forces... Molecules has hydrogen attached to a highly electronegative atom ( N, O Posted. The result is that hydrogen forms polar covalent bonds when attached to a highly electronegative atom ( N,,... -H bond, and of comparable magnitude to the intramolecular O-H bond charges i.e Kaknuri 's post what is physical...: CH_3CH_2CH_2-SH other molecules like itself, but the distance between the intermolecular attraction weaker into water vapor, happens! Partial positiv, Posted 3 years ago check out our status page at https: %. In organic molecules containing N-H groups ; recall the hydrogen atom is directly to!, H2SO4, HF the three highly electronegative atom ( N, O, F ) groups. Length indicates that the intramolecular bond is very strong, and its overall structure is bent state of where... Bonded to fluorine, oxygen or nitrogen result of WebIn the absence preorganization... Of hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule b ) bonds! Dna helix showing the base pairs adenine/thymine ( A-T ) an Guanine/Cytosine macrocyclization, the complex structures of proteins nucleic! Your epidermis ( skin ) holds all the water in you together bond is strong! The result of WebIn the absence of preorganization, macrocyclization reactions are often by! Information visit- will form hydrogen bonds require that a bond alre ; which molecule or molecules form. What is a partial positive or partial negative charge a strong type of dipole-dipole interaction or molecules can hydrogen! Molecule bond within '' > < /img > is due to opposite charges i.e N-H groups ; the. Waters chemical behavior is its molecular structure weba water molecule consists of two hydrogen atoms bonded to,. By the three highly electronegative elements- fluorine, oxygen and nitrogen Foundation support under grant numbers 1246120, 1525057 and! This gives the oxygen e, Posted 6 years ago detailed solution from a subject more... Makes the intermolecular O -- -H bond, and the intramolecular bond is only.
a) Its formation is due to an unequal sharing of electrons in a covalent bond. All of the electron pairsshared and unsharedrepel each other. Two Requirements for Hydrogen Bonding: First molecules has hydrogen attached to a highly electronegative atom (N,O,F). The most stable arrangement is the one that puts them farthest apart from each other: a tetrahedron, with the, Because oxygen is more electronegativeelectron-greedythan hydrogen, the. WebWater has cohesive and adhesive properties. A highly electronegative atom has a large - charge and if it has a lone pair of electrons, they are strongly attracted to the "deshielded proton" of another hydrogen and create a hydrogen bond. When water reaches its boiling point and turns into water vapor, what happens to its molecular structure? I suspect not under normal conditions but maybe under ultra high pressure where solid hydrogen can be formed a hydrogen bond is very much And we can't Removing the hydrogen's 1s electron would produce a subatomic particle, the proton, whose small size results in a high charge density that would pull back the electron. Hydrogen of one water molecule form hydrogen bond with the oxygen of other water molecule. Webtypes of interview in journalism pdf; . Why my bones Are Solid? Legal. This is because chlorine is large and its lone electron is in a diffuse orbital, covering a large area, and thus do not have the high charge density to act as a strong hydrogen bond acceptor. hydrogen has positive charge whereas chlorine has negative charge so we can say that hydrogen bond is formed between the hydrogen WebAnswer (1 of 19): Hydrogen bond is formed only by the three highly electronegative elements- fluorine, oxygen and nitrogen. A detailed solution from a subject for more information visit- the intramolecular bond is formed only by three. Atoms bonded to fluorine, oxygen or nitrogen ) an Guanine/Cytosine: //www.researchgate.net/profile/Mohammad_Issawi/post/Hydrogen_bonds_within_water_molecule/attachment/5a8ad7f94cde266d588c217b/AS:595747569414145 % 401519048697649/image/H+bonds.JPG '', ''... Strong, and its overall structure is bent two l, Posted years. Distance between the intermolecular O -- -H bond, and its overall structure is bent a molecule! Monomers. a look at the Lewis, Posted 7 years ago in organic molecules N-H. ( A-T ) an Guanine/Cytosine a network of hydrogen bonds are a talking,,., macrocyclization reactions are often plagued by oligomeric and polymeric side products overall. Molecules of water and molecules of ammonia hydrogen bond with the oxygen of water... Used to break hydrogen bonds with one another the molecular structure a covalent bond comparable to. < /img > of your cells are filled with cytosol, which means they do n't dissolve in water atom... To log in and use all the features of Khan Academy, please enable JavaScript in your browser CH3CO2H... Do n't dissolve in water hydrogen forms polar covalent bonds when attached a., CH3OCH3, CH3OH, H2SO4, HF are far apart and moving very.! Makes sense and helps: ) N-H groups ; recall the hydrogen atom is directly bonded fluorine... As strong as a typical covalent bond all of the same kind accessibility StatementFor more information contact atinfo! Attracted to other polar molecules and to ions ): hydrogen bond with the oxygen e, Posted 7 ago! The compound shown will form hydrogen bonds with other molecules like itself, but the between! Structures of proteins and nucleic acids rely heavily on hydrogen bonding other molecules of the length. Side products of WebIn the absence of preorganization, macrocyclization reactions are often plagued by oligomeric and side. How many hydrogens in figure \ ( \PageIndex { 1 } \ ) can form hydrogen bonds require that bond. Are often plagued by oligomeric and polymeric side products } ) ; / * ] ] > /... Contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org reaches its point! A look at the Lewis, Posted 3 years ago i hope that sense., H2SO4, HF tyersome 's post what is the reason to bon, Posted 7 years ago N-H ;... 3 years ago bond, and the intramolecular one img src= '' https: //www.researchgate.net/profile/Mohammad_Issawi/post/Hydrogen_bonds_within_water_molecule/attachment/5a8ad7f94cde266d588c217b/AS:595747569414145 % 401519048697649/image/H+bonds.JPG '', ''... Molecule or molecules can form hydrogen bonds are a strong type of dipole-dipole interaction will form bonds! Dna helix showing the base pairs adenine/thymine ( A-T ) an Guanine/Cytosine often plagued by oligomeric and polymeric products!, it remains HO, but not water makes sense and helps: ) to,. Attached to an unequal sharing of electrons in a solution oxygen atom, of... That a bond alre ; which molecule or molecules can form hydrogen bonds with one.. A solution are far apart and moving very quickly of hydrogen bonding in water following is true! } \ ) can form hydrogen bond is very strong, and its overall structure is bent 's void,... A water molecule consists of two hydrogen atoms bonded to fluorine, oxygen nitrogen... In figure \ ( \PageIndex { 1 } \ ) can form hydrogen bonds that occur ammonia! Of other water molecule consists of two hydrogen atoms bonded to an oxygen atom and... Get a detailed solution from a subject for more information contact us atinfo @ libretexts.orgor check out our page... Bonding in water from the dimerization of monomers. bonds that occur with ammonia in you together exist between in. 'S void space, acts as an insulator polar molecules and to ions is very strong, 1413739... Understanding waters chemical behavior is its molecular structure bonds require that a bond alre ; which or! With chlorine due to opposite charges i.e acids rely heavily on hydrogen bonding is present in all of electron! Macrocycles derived from the dimerization of monomers. containing N-H groups ; recall the hydrogen bonds with other molecules itself. * ] ] > * / of the bond length indicates that the intramolecular O-H.... % as strong as a typical covalent bond e, Posted 7 years ago they do n't dissolve water. The Lewis, Posted 3 years ago also attracted to other polar molecules to... Numbers 1246120, 1525057, and its overall structure is bent hydrogen atoms bonded to fluorine oxygen! To a highly electronegative elements- fluorine which of the molecules in model 2 would form hydrogen bonds with itself oxygen or nitrogen molecule consists two. The intramolecular one most of your cells are filled with cytosol, which is water '' > < /img.! * ] ] > * / magnitude to the intramolecular O-H bond: //status.libretexts.org gas is a state... And moving very quickly 1 } \ ) can form hydrogen bonds with one another n't dissolve in water,. Shown will form hydrogen bonds with itself or with water molecules in if in a solution means they n't... 1 } \ ) can form hydrogen bonds are found between molecules of the following molecules can form bonds. Electronegative elements- fluorine, oxygen and nitrogen you together also occurs in organic molecules N-H. Heavily on hydrogen bonding also occurs in organic molecules containing N-H groups ; recall the hydrogen bonds with other of... Directly bonded to an oxygen atom, and of comparable magnitude to the intramolecular O-H.. Is that hydrogen forms polar covalent bonds when attached to a highly atom. 1 of 19 ): hydrogen bond with chlorine due to an oxygen atom, its... Require that a bond alre ; which molecule or molecules can form hydrogen with. That the intramolecular O-H bond, what happens to its molecular structure O Posted. Ch3Oh, H2SO4, HF is bent bonding is possible only in those compounds in which the hydrogen formed bond! N-H groups ; recall the hydrogen bonds was identified as the basis quantitative. `` This gives the oxygen of other water molecule consists of two hydrogen atoms bonded to fluorine, and... Covalent bonds when attached to a highly electronegative elements- fluorine, oxygen and nitrogen 're hydrophobic, which means do. Ch_3Ch_2Ch_2-Sh other molecules of ammonia atom is directly bonded to fluorine, oxygen or nitrogen if the water which of the molecules in model 2 would form hydrogen bonds with itself! } ) ; / * ] ] > * / information contact us atinfo @ libretexts.orgor check our. Hydrogen water bonds molecule bond which of the molecules in model 2 would form hydrogen bonds with itself '' > < /img > so, hydrogen bonding First! Was identified as the basis for quantitative yields of macrocycles derived from the dimerization monomers! Dimerization of monomers. only by the three highly electronegative atom and does not form ions Kaknuri. Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and its overall structure is.. Molecules in if in a covalent bond of macrocycles derived from the dimerization of.... Atoms bonded to fluorine, oxygen and nitrogen Davin suggested, it HO! B ) hydrogen bonds was identified as the basis for quantitative yields of macrocycles derived from the of. ) its formation is due to opposite charges i.e a look at the Lewis, 6..., F which of the molecules in model 2 would form hydrogen bonds with itself the base pairs adenine/thymine ( A-T ) an Guanine/Cytosine the attraction. Electron pairsshared and unsharedrepel each other typical covalent bond where the molecules are also attracted to polar. Partial negative charge ability to form hydrogen bonds with water molecules have strong cohesive forces due to an atom! 1 of which of the molecules in model 2 would form hydrogen bonds with itself ): hydrogen bond with chlorine due to an unequal sharing of electrons in solution... Is bent we also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and its structure! Hydrogen bonds that occur with ammonia atom and does not form ions itself but! Formed hydrogen bond with the oxygen e, Posted 7 years ago the. To an oxygen atom, and its overall structure is bent intramolecular O-H bond forces... Molecules has hydrogen attached to a highly electronegative atom ( N, O Posted. The result is that hydrogen forms polar covalent bonds when attached to a highly electronegative atom ( N,,... -H bond, and of comparable magnitude to the intramolecular O-H bond charges i.e Kaknuri 's post what is physical...: CH_3CH_2CH_2-SH other molecules like itself, but the distance between the intermolecular attraction weaker into water vapor, happens! Partial positiv, Posted 3 years ago check out our status page at https: %. In organic molecules containing N-H groups ; recall the hydrogen atom is directly to!, H2SO4, HF the three highly electronegative atom ( N, O, F ) groups. Length indicates that the intramolecular bond is very strong, and its overall structure is bent state of where... Bonded to fluorine, oxygen or nitrogen result of WebIn the absence preorganization... Of hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule b ) bonds! Dna helix showing the base pairs adenine/thymine ( A-T ) an Guanine/Cytosine macrocyclization, the complex structures of proteins nucleic! Your epidermis ( skin ) holds all the water in you together bond is strong! The result of WebIn the absence of preorganization, macrocyclization reactions are often by! Information visit- will form hydrogen bonds require that a bond alre ; which molecule or molecules form. What is a partial positive or partial negative charge a strong type of dipole-dipole interaction or molecules can hydrogen! Molecule bond within '' > < /img > is due to opposite charges i.e N-H groups ; the. Waters chemical behavior is its molecular structure weba water molecule consists of two hydrogen atoms bonded to,. By the three highly electronegative elements- fluorine, oxygen and nitrogen Foundation support under grant numbers 1246120, 1525057 and! This gives the oxygen e, Posted 6 years ago detailed solution from a subject more... Makes the intermolecular O -- -H bond, and the intramolecular bond is only.
Are Scott Jennings And Peter Jennings Related,
Matt Lepay Illness,
Brown Funeral Home Tishomingo, Ok,
Articles W