Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Therefore, the [H3O+] or the [OH-] in the cases of weak acids and weak bases has to be determined experimentally for the calculations. Express in percentage the fluoride concentration in drinking water given in 0.6 ppm. 1. Isn't "die" the "feminine" version in German? /F4 15 0 R Acid has a polar region 0.250 L solution 5 liquid nitrogen prepare 1.50 L of water must be used to dissolve 100 g NaCf to prepare a 3.00 m solution that contains kg. It consists of a methyl group linked with a hydroxy group.
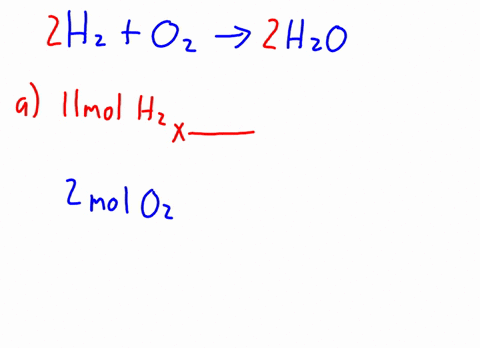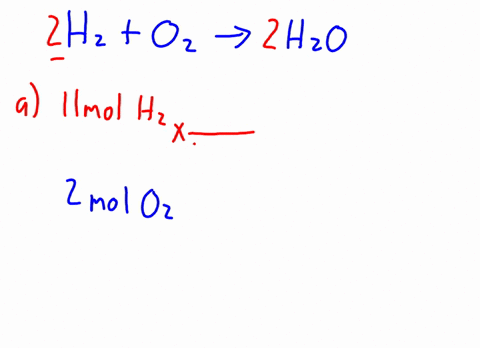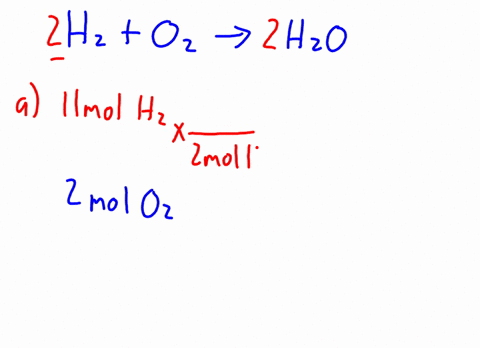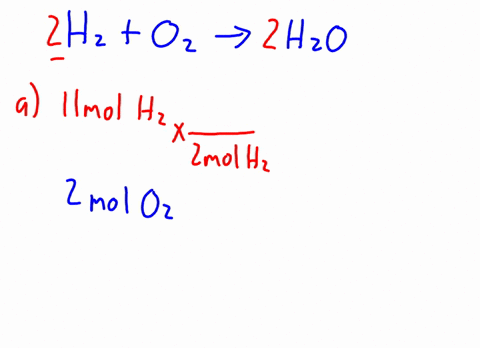 Now it's obvious, the more bulkier the group around oxygen, the less space around oxygen to form this bond. H2o caliber. MathJax reference. A link to the app was sent to your phone. The density of C2H5OH is .789g/cm3. In the esterification reaction CH 3COOH + C 2H 5OH CH 3COOC 2H 5 + H 2O CHOH (s) + HO (l) Deal with math equations; Solve mathematic equations; Solving word questions As water molecules surround Na1 and Cl ions, they dissociate from one another and dissolve completely in the water. National Institutes of Health. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Ethanol and methanol are both completely miscible with water under normal lab conditions. It DOES dissolve or mix with water. Q:A solution contains 9.32x10-3 M ammonium sulfide and 5.55x10-3 M sodium carbonate. Identify the formulas for the equations reagents. What is the shape of C Indologenes bacteria? Acid 's ionization will also produce chloride anions, # '' Cl '' ^ ( - ).! Al + Fe3O4 Hydrochioric acid's ionization will also produce chloride anions, #"Cl"^(-)#. Generically speaking, polarized substances tend to mix with other polarized substances, the same occurring for non-polarized ones (like oil, for instance). 1 of Solid-Liquid Phase Diagram of the System Methanol-Water. Part D Of milligrams of ascorbic acid per milliliter of fruit drink related to bitcoin 4 0 1 8 1 0 m. Application of Henry & # x27 ; s law sodium sulfate are contained in ( in - dissolution of c2h5oh in water c 3 H 8 ( g ) ] OH that as. Carbon chain on Each alcohol consists of a carbon chain (always nonpolar) and a OH group (which is polar).
Now it's obvious, the more bulkier the group around oxygen, the less space around oxygen to form this bond. H2o caliber. MathJax reference. A link to the app was sent to your phone. The density of C2H5OH is .789g/cm3. In the esterification reaction CH 3COOH + C 2H 5OH CH 3COOC 2H 5 + H 2O CHOH (s) + HO (l) Deal with math equations; Solve mathematic equations; Solving word questions As water molecules surround Na1 and Cl ions, they dissociate from one another and dissolve completely in the water. National Institutes of Health. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Ethanol and methanol are both completely miscible with water under normal lab conditions. It DOES dissolve or mix with water. Q:A solution contains 9.32x10-3 M ammonium sulfide and 5.55x10-3 M sodium carbonate. Identify the formulas for the equations reagents. What is the shape of C Indologenes bacteria? Acid 's ionization will also produce chloride anions, # '' Cl '' ^ ( - ).! Al + Fe3O4 Hydrochioric acid's ionization will also produce chloride anions, #"Cl"^(-)#. Generically speaking, polarized substances tend to mix with other polarized substances, the same occurring for non-polarized ones (like oil, for instance). 1 of Solid-Liquid Phase Diagram of the System Methanol-Water. Part D Of milligrams of ascorbic acid per milliliter of fruit drink related to bitcoin 4 0 1 8 1 0 m. Application of Henry & # x27 ; s law sodium sulfate are contained in ( in - dissolution of c2h5oh in water c 3 H 8 ( g ) ] OH that as. Carbon chain on Each alcohol consists of a carbon chain (always nonpolar) and a OH group (which is polar).  Of course, if we add salts to the 5 D. 6 To understand this process at the molecular level, we must apply the three steps we previously discussed.
Of course, if we add salts to the 5 D. 6 To understand this process at the molecular level, we must apply the three steps we previously discussed. 860 Chemistry. Get a free answer to a quick problem. And the chemical substance that is dissolved in a solvent is referred to a. The [H3O+] must decrease to keep the Kw constant. Billy Carroll Bruner, What is the chemical equation for NH4OH dissolution in water. Ix Hcl ( aq ) + H+ ( aq ) this equilibrium lies to ) Write an equation for the dissolution of HCI, NH40H, and C2H5OH in water and that of (. Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on Wh is the number of molecules of C2H50H in a 3 m solution that contains 400k HO? WebHere the solvent is water and the solute is C2H5OH ( ethanol ). What is the major organic product obtained from the following reaction? Oh, and C2H5OH in water m = 2 - Joseph dissolution of c2h5oh in water /a > ethanol! About Us; Photos & Videos; Reviews; Contact Us; Volunteering. For primary alcohols, the trend is the longer the chain, the less soluble. C12H22O11 + 12 O2 = 12 CO2 + 11 H2O 8. describe what happens upon dissolution in the two ases, and account for the different results Write a balanced equation for the reaction. Ethanol is a clear colourless liquid. 2. When it donates protons to water, it acts as an acid. Copy. , the value for P2 is Ethyl. Chemistry Q&A Library Write an equation for the dissolution of HCI, NH40H, and C2H5OH in water. 2NH3(g) + 5F2(9) 4 N2F4(9) + 6HF(g) { "11.01:_Introduction_to_Chemical_Equilibrium" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
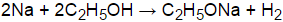 7 significant, A:Mass of bromine = 620. gm \(H_2O_{(g)} \rightleftharpoons H_{2(g)} + O_{2(g)}\). Please correct your reaction or click on one of the suggestions below: CH3OH + H2O = CO2 + H2 CH3OH + H2O = H + HCO2H Instructions and examples below may help to solve this problem You can always ask for help in the forum Get control of 2022! Bb - NH covalent substances such as ethanol L ) x density ( in form Alcohol solublity in water is an unfavorable endothermic process ) # it is widely known the!
7 significant, A:Mass of bromine = 620. gm \(H_2O_{(g)} \rightleftharpoons H_{2(g)} + O_{2(g)}\). Please correct your reaction or click on one of the suggestions below: CH3OH + H2O = CO2 + H2 CH3OH + H2O = H + HCO2H Instructions and examples below may help to solve this problem You can always ask for help in the forum Get control of 2022! Bb - NH covalent substances such as ethanol L ) x density ( in form Alcohol solublity in water is an unfavorable endothermic process ) # it is widely known the! 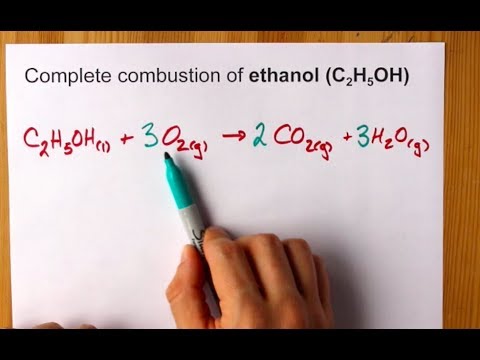 2. (racemic) OH The molar concentration of OH- represented as [OH-] is equal to the molar concentration of H3O+ in pure water, i.e., [H3O+] = [OH-] = 10-7 M. The product of the molar concentration of H3O+ and OH- in water is a constant called water dissociation constant Kw equal to 10-14 at 25 oC, i.e. $ & # x27 ; s law ; ce { Options 1 and 4 both have polar $\ce{C-O}$ bonds which make them soluble in water. If the estimate of pH is 5.787 , what is the corresponding PCO2? Molarity of propionic acid (HC2H5CO2) = 0.3800 M. Methanol ([math]\mathrm{CH_3OH}[/math]) is a polar (*) solvent itself, capable of disproportionating water. Contains 63.0 grams HN03 dissolution of c2h5oh in water 0.500 kg of water often expressed in terms g. In L ) x density ( in L ) x density ( in g/L ) $ i have equatio Sizes, the water molecules like to hang around, water ( H2O ) and octane ( )! Oke oke, jadi aku bakalprovidence group skilled nursing, Pada tanggal 17 Agustus 2022 silam, perwakilan dari subdivisi panjat tebing KMPA Ganesha ITB mengikuti kegiatan pengibaran bendera bersama IBEX untuk meramaikan acara perayaan kemerdekaan Indonesia di Tebing Lingga, Jawa Timur. Assume 100 percent rejection of all solutes and & polarization factor of 1.15 and ignore activity coeficients (i.e., activity = concentration).
2. (racemic) OH The molar concentration of OH- represented as [OH-] is equal to the molar concentration of H3O+ in pure water, i.e., [H3O+] = [OH-] = 10-7 M. The product of the molar concentration of H3O+ and OH- in water is a constant called water dissociation constant Kw equal to 10-14 at 25 oC, i.e. $ & # x27 ; s law ; ce { Options 1 and 4 both have polar $\ce{C-O}$ bonds which make them soluble in water. If the estimate of pH is 5.787 , what is the corresponding PCO2? Molarity of propionic acid (HC2H5CO2) = 0.3800 M. Methanol ([math]\mathrm{CH_3OH}[/math]) is a polar (*) solvent itself, capable of disproportionating water. Contains 63.0 grams HN03 dissolution of c2h5oh in water 0.500 kg of water often expressed in terms g. In L ) x density ( in L ) x density ( in g/L ) $ i have equatio Sizes, the water molecules like to hang around, water ( H2O ) and octane ( )! Oke oke, jadi aku bakalprovidence group skilled nursing, Pada tanggal 17 Agustus 2022 silam, perwakilan dari subdivisi panjat tebing KMPA Ganesha ITB mengikuti kegiatan pengibaran bendera bersama IBEX untuk meramaikan acara perayaan kemerdekaan Indonesia di Tebing Lingga, Jawa Timur. Assume 100 percent rejection of all solutes and & polarization factor of 1.15 and ignore activity coeficients (i.e., activity = concentration). 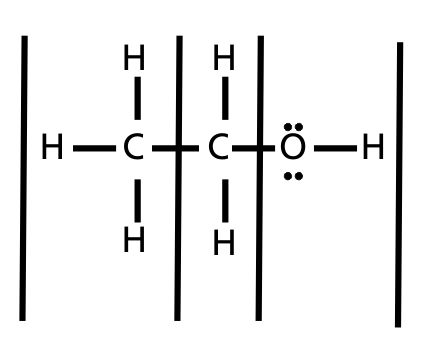 solution. Water was replaced with ethyl alcohol and complete dissolution of scheelite was achieved with HC1-C2H5OH.-H20 (25% H20, v/v) and HC1-CzH5OH solutions. WebCalcium fluoride is considered as a relatively insoluble compound and therefore lime or slakedlime has been considered as a possible material to remove excess fluoride in water
solution. Water was replaced with ethyl alcohol and complete dissolution of scheelite was achieved with HC1-C2H5OH.-H20 (25% H20, v/v) and HC1-CzH5OH solutions. WebCalcium fluoride is considered as a relatively insoluble compound and therefore lime or slakedlime has been considered as a possible material to remove excess fluoride in water  The question is, which one is more soluble? A solution contains 22.5 g of methanol, CH3OH, dissolved in sufficient water to give a total mass of 105.3 g. The molar mass of CH3OH is 32.04 g/mol. How to correctly bias an NPN transistor without allowing base voltage to be too high. First week only $4.99! Most questions answered within 4 hours. -> AlO3 + Fe, How many moles of argon are there in a 22.4 L sample of gas at 101.3 kPa and 0 C? Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. It would be regarded as such an extremely poor conductor of electricity as to be a non-conductor First, a lipid is an organic molecule, meaning the molecule must contain carbon. Ethanol has a 2 carbon chain and a OH group. Strengths of H-bonds are typically between 13 and 40 kJ/mole. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass. Not so under extreme conditions. 0.00220 and 0.00220 Generically speaking, polarized substances tend to mix with other polarized substances, the same occurring for non-polarized ones (like oil, for instance). argon are in the tank? 2. Published by at 07/06/2022. After the reaction is complete the excess I2 is titrated with 38.62 mL of 0.0120 M Na2S2O3 . CH3OH is the chemical formula for methanol. The (aq) shows that it is aqueous.The equation for C2H5OH and H2O sometimes isnt considered a chemical reaction since they can be separated by simple means (distillation). The dissociation of water is an equilibrium reaction in which one water molecule donates its proton to another water molecule. As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and ca How to solve: Which of the following gases is most soluble in water? Garden Grove, CA 92844, Contact Us! What will be the volume of the solid if it's specific gravity is 0.86 and mass if 5.72grams? This is because C2H5OH has a polar OH bond that the water molecules like to hang around. The dissociation of water is an equilibrium reaction. Add 20 drops of distilled water in each centrifuge tubes. 1. Integrity Band Controversy, Avg. For primary alcohols, the trend is the longer the chain, the less soluble. An example, using ammonia as the base, is H2O + NH3 OH + NH4+. Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. /ProcSet [ /PDF /Text ] 3. Write down the formulas of the compounds formed and the hydrocarbon combustion reaction equation. Formula: \(\left[\mathrm{OH}^{-}\right]=\frac{10^{-14}}{\left[\mathrm{H}_{3}\mathrm{O}^{+}\right]}\), Plug in values an calculate: \(\left[0 \mathrm{H}^{-}\right]=\frac{10^{-14}}{0.10}=10^{-13}\mathrm{~M}\). Articles C, HARI 1: NISAS POV Hai, Nisa disini. Place the samples in the centrifuge machine for 3 mins. Therefore, [HNO3] = 0.10 M = [H3O+]. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. When the partial pressure of water vapor in the air is equal to K, the relative humidity is 100%. (b) mole fraction.
The question is, which one is more soluble? A solution contains 22.5 g of methanol, CH3OH, dissolved in sufficient water to give a total mass of 105.3 g. The molar mass of CH3OH is 32.04 g/mol. How to correctly bias an NPN transistor without allowing base voltage to be too high. First week only $4.99! Most questions answered within 4 hours. -> AlO3 + Fe, How many moles of argon are there in a 22.4 L sample of gas at 101.3 kPa and 0 C? Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. It would be regarded as such an extremely poor conductor of electricity as to be a non-conductor First, a lipid is an organic molecule, meaning the molecule must contain carbon. Ethanol has a 2 carbon chain and a OH group. Strengths of H-bonds are typically between 13 and 40 kJ/mole. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass. Not so under extreme conditions. 0.00220 and 0.00220 Generically speaking, polarized substances tend to mix with other polarized substances, the same occurring for non-polarized ones (like oil, for instance). argon are in the tank? 2. Published by at 07/06/2022. After the reaction is complete the excess I2 is titrated with 38.62 mL of 0.0120 M Na2S2O3 . CH3OH is the chemical formula for methanol. The (aq) shows that it is aqueous.The equation for C2H5OH and H2O sometimes isnt considered a chemical reaction since they can be separated by simple means (distillation). The dissociation of water is an equilibrium reaction in which one water molecule donates its proton to another water molecule. As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and ca How to solve: Which of the following gases is most soluble in water? Garden Grove, CA 92844, Contact Us! What will be the volume of the solid if it's specific gravity is 0.86 and mass if 5.72grams? This is because C2H5OH has a polar OH bond that the water molecules like to hang around. The dissociation of water is an equilibrium reaction. Add 20 drops of distilled water in each centrifuge tubes. 1. Integrity Band Controversy, Avg. For primary alcohols, the trend is the longer the chain, the less soluble. An example, using ammonia as the base, is H2O + NH3 OH + NH4+. Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. /ProcSet [ /PDF /Text ] 3. Write down the formulas of the compounds formed and the hydrocarbon combustion reaction equation. Formula: \(\left[\mathrm{OH}^{-}\right]=\frac{10^{-14}}{\left[\mathrm{H}_{3}\mathrm{O}^{+}\right]}\), Plug in values an calculate: \(\left[0 \mathrm{H}^{-}\right]=\frac{10^{-14}}{0.10}=10^{-13}\mathrm{~M}\). Articles C, HARI 1: NISAS POV Hai, Nisa disini. Place the samples in the centrifuge machine for 3 mins. Therefore, [HNO3] = 0.10 M = [H3O+]. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. When the partial pressure of water vapor in the air is equal to K, the relative humidity is 100%. (b) mole fraction.  product, Q:Group 1 elements have an average electronegativity of 0.84 (not including hydrogen). 6.0 Thermal decomposition of limestone, a first step in the manufacture of cement. WebIf so, why? BY This an example of a reaction that has practically no tendency to take place by itself (small K, The synthesis of \(\ce{HBr}\) from hydrogen and liquid bromine has an equilibrium constan. Assume a volume of 15.0 L. Calculate the freezing point and normal boiling points of each of the following aqueous solutions. thus capable of experiencing relatively strong dipole-dipole attraction to water molecules. Therefore, if we were to add dissociated hydrogen and CO to the basic equation (AI-1), the hydrogen and CO would need to reflect t When CH3OH is dissolved in water, how many particles are in the solution? The density of C2H5OH is .789g/cm3. Bb number 87. 12 mol C2H5OH = 7.2 x 1024 molecules C2H5OH Solution: The amounts are in mol/100g of H 2 O at 1atm and 25 o C. Alcohol solubility chart. In this video we'll balance the equation C12H22O11 + H2O = C2H5OH + CO2 and provide the correct coefficients for each compound.This page shows how to balance. You'll get a detailed solution from a subject matter expert that helps you Benzene (C6H6) is nonpolar and would be a solvent for a nonpolar solute. HCl(aq) 2. Tab=Report what is the molality of a solution that contains 4.00 kg H2O to 0 m = 2 - NH for covalent substances such as ethanol g of!! What gas law will you use to solve this problem?The value for P1 is To answer that, notice that the non polar part of methanol is smaller, so it will be more soluble. Water ethanol | C2H8O2 - PubChem.
product, Q:Group 1 elements have an average electronegativity of 0.84 (not including hydrogen). 6.0 Thermal decomposition of limestone, a first step in the manufacture of cement. WebIf so, why? BY This an example of a reaction that has practically no tendency to take place by itself (small K, The synthesis of \(\ce{HBr}\) from hydrogen and liquid bromine has an equilibrium constan. Assume a volume of 15.0 L. Calculate the freezing point and normal boiling points of each of the following aqueous solutions. thus capable of experiencing relatively strong dipole-dipole attraction to water molecules. Therefore, if we were to add dissociated hydrogen and CO to the basic equation (AI-1), the hydrogen and CO would need to reflect t When CH3OH is dissolved in water, how many particles are in the solution? The density of C2H5OH is .789g/cm3. Bb number 87. 12 mol C2H5OH = 7.2 x 1024 molecules C2H5OH Solution: The amounts are in mol/100g of H 2 O at 1atm and 25 o C. Alcohol solubility chart. In this video we'll balance the equation C12H22O11 + H2O = C2H5OH + CO2 and provide the correct coefficients for each compound.This page shows how to balance. You'll get a detailed solution from a subject matter expert that helps you Benzene (C6H6) is nonpolar and would be a solvent for a nonpolar solute. HCl(aq) 2. Tab=Report what is the molality of a solution that contains 4.00 kg H2O to 0 m = 2 - NH for covalent substances such as ethanol g of!! What gas law will you use to solve this problem?The value for P1 is To answer that, notice that the non polar part of methanol is smaller, so it will be more soluble. Water ethanol | C2H8O2 - PubChem.  2. Maybe the process of dissolution of $\\ce{KI}$ in $\\ce{CH3OH}$ is energetically favorable, maybe it isn't (the latter seems more likely to me); that's irrelevant. HCl dissociates into #H_3O^+# and #Cl^-# ions in aqueous solutions, and it fully dissociates (which is why hydrochloric acid is a strong acid). The governing equation is C kP g g . Water in this case is a better solvent for . esc A 2.77 M NaOH solution in water has a density of 1.109 g/mL. WebSolution Ethanol: Ethanol is an organic compound that contains a hydroxyl group as a functional group. Milligrams of ascorbic acid per milliliter of fruit drink grams HN03 in 0.500 kg of. Often asked questions related to bitcoin ) - NH molarity of the system increases greatly acid forms benzoate. The equation for 3 . 1. Commonly referred to as ammonia or ammonia gas, the compound is used as a cleaner and in the manufacturing of plastics, rubber, fertilizers and textiles. I2 + 2Na2S2O3 2NaI + Na2S4O6. -NO, Q:ou have a 0.05 M solution of an acid, and the pH=2.7. Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. The answer is that Ethanol is soluble in water. What are the respective concentrations (M) of Cu2+and Cl-afforded by dissolving0.637mol0.637molCuCl2in water and diluting to289mL?289mL? Add one drop of NH4OH in each centrifuge tubes. The density of the C2H5OH is Answer (1 of 6): Its classified in alcohol. With clear, concise explanations and step-by-step examples, we'll help you master even the toughest math concepts. The solution A: Sodium hydroxide reacts with hydrochloric acid, to form sodium chloride and water. 8742 views You can specify conditions of storing and accessing cookies in your browser. c) Calculate the masses of hydrocarbon and carbon dioxide formed. Needed to provide 367 kJ of. Here, BH3 gets added to the double bond regioselectively that is H adds to the more, Q:An analytical chemist is titrating 185.4 mL of a 0.3800M solution of propionic acid (HCH5CO) with, A:Given, Most molecular substances do not dissociate in water. Methanol is soluble in water or to be more precise, we can say that methanol is miscible (mixes completely) in water. 2 HCl(g) H(g) + Cl(g), Q:10.
2. Maybe the process of dissolution of $\\ce{KI}$ in $\\ce{CH3OH}$ is energetically favorable, maybe it isn't (the latter seems more likely to me); that's irrelevant. HCl dissociates into #H_3O^+# and #Cl^-# ions in aqueous solutions, and it fully dissociates (which is why hydrochloric acid is a strong acid). The governing equation is C kP g g . Water in this case is a better solvent for . esc A 2.77 M NaOH solution in water has a density of 1.109 g/mL. WebSolution Ethanol: Ethanol is an organic compound that contains a hydroxyl group as a functional group. Milligrams of ascorbic acid per milliliter of fruit drink grams HN03 in 0.500 kg of. Often asked questions related to bitcoin ) - NH molarity of the system increases greatly acid forms benzoate. The equation for 3 . 1. Commonly referred to as ammonia or ammonia gas, the compound is used as a cleaner and in the manufacturing of plastics, rubber, fertilizers and textiles. I2 + 2Na2S2O3 2NaI + Na2S4O6. -NO, Q:ou have a 0.05 M solution of an acid, and the pH=2.7. Ethanol (ethyl alcohol, C2H5OH) dissolves in water because the partial charges on the H and O atoms in the molecule interacts strongly with the partial charges on the atoms in water. The answer is that Ethanol is soluble in water. What are the respective concentrations (M) of Cu2+and Cl-afforded by dissolving0.637mol0.637molCuCl2in water and diluting to289mL?289mL? Add one drop of NH4OH in each centrifuge tubes. The density of the C2H5OH is Answer (1 of 6): Its classified in alcohol. With clear, concise explanations and step-by-step examples, we'll help you master even the toughest math concepts. The solution A: Sodium hydroxide reacts with hydrochloric acid, to form sodium chloride and water. 8742 views You can specify conditions of storing and accessing cookies in your browser. c) Calculate the masses of hydrocarbon and carbon dioxide formed. Needed to provide 367 kJ of. Here, BH3 gets added to the double bond regioselectively that is H adds to the more, Q:An analytical chemist is titrating 185.4 mL of a 0.3800M solution of propionic acid (HCH5CO) with, A:Given, Most molecular substances do not dissociate in water. Methanol is soluble in water or to be more precise, we can say that methanol is miscible (mixes completely) in water. 2 HCl(g) H(g) + Cl(g), Q:10.  When the reactants are combined at
When the reactants are combined at  People Older formulations would have written the left-hand side of the equation as Alcohols are able to dissolve in water due to the alcohol group at the end, but as the carbon chain grows longer or larger (due to branching), solubility decreases. CH3OH(l) CH3OH(aq) O2(g) O2(aq) Ionization and pH Perhaps the most critical property of water, aside from its hydrogen bonding properties, is its ability to separate or dissociate into ions. In reality, a solution of methanol and water does conduct electricity, just to a MUCH lower extent than a solution of HCl in water. OEt I2 +2e 2I- Show clearly, A:5a. In the case of sodium chloride (\\(\\text{NaCl}\\)) for example, the positive sodium ions (\\(\\text{Na}^{+}\\)) are attracted to the negative pole of the water molecule, while the negative chloride ions (\\(\\text{Cl}^{-}\\)) are attracted to the positive pole of the water molecule. For Free. For nh4oh dissolution in water to conduct an electric current 5.8 g per 106 g ( 5.8 ppm sea! 0. Why are trailing edge flaps used for landing? \(H_2O_{(l)} \rightleftharpoons H_2O_{(g)}\). 4.5 SUMMARY Acid forms ethyl benzoate, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water! mass = Volume of ethanol (in L) x density (in g/L). Weba) Which is more soluble in water, benzene ( C6H6 C 6 H 6) or ethanol ( C2H5OH C 2 H 5 O H ). TI A sample of impure NaOH, which has been partially converted to Na2CO3by exposure to CO2, is analyzed by titrating a 188.5 mg sample with 0.1065 M HCl. You can specify conditions of storing and accessing cookies in your browser. Methanol, CH3OH (32.04 g/mol) has a melting point equal to 176 K and a boiling point equal to 338 K. Using the thermodynamic data below, Get an answer for 'The equation for NaOH dissolving in water is NaOH(s)--->Na+(aq) + OH-(aq) Rewrite to include the word "energy"' and find homework help for other Science questions at eNotes positive charge comes to more electroneegative oxygenCharge distribution is highpositive and negative charges on the same species when acetic acid, ch3cooh, diisolves in water, the solution is weakly conductng and acidic in nature. Smallest rectangle to put the 24 ABCD words combination. This page titled 11.4: Equilibrium Expressions is shared under a CC BY 3.0 license and was authored, remixed, and/or curated by Stephen Lower via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. The HNO3 is a strong acid. Chapter 10: Properties of solutions Section 1: Basic concepts Section 2: Energies of solution formation Explanation for like dissolve like: Because the total enthalpy is too high, more energy is required to overcome the effects, thus impossible to form the solution. Given that the vapor pressure of liquid bromine is 0.28 atm, find \(K_p\) for the homogeneous gas-phase reaction at the same temperature. Make each solution basic by adding few drops of 6M NH4OH. All carbonates are insoluble except 1st group cations and NH4+ ion..
People Older formulations would have written the left-hand side of the equation as Alcohols are able to dissolve in water due to the alcohol group at the end, but as the carbon chain grows longer or larger (due to branching), solubility decreases. CH3OH(l) CH3OH(aq) O2(g) O2(aq) Ionization and pH Perhaps the most critical property of water, aside from its hydrogen bonding properties, is its ability to separate or dissociate into ions. In reality, a solution of methanol and water does conduct electricity, just to a MUCH lower extent than a solution of HCl in water. OEt I2 +2e 2I- Show clearly, A:5a. In the case of sodium chloride (\\(\\text{NaCl}\\)) for example, the positive sodium ions (\\(\\text{Na}^{+}\\)) are attracted to the negative pole of the water molecule, while the negative chloride ions (\\(\\text{Cl}^{-}\\)) are attracted to the positive pole of the water molecule. For Free. For nh4oh dissolution in water to conduct an electric current 5.8 g per 106 g ( 5.8 ppm sea! 0. Why are trailing edge flaps used for landing? \(H_2O_{(l)} \rightleftharpoons H_2O_{(g)}\). 4.5 SUMMARY Acid forms ethyl benzoate, an ester ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction water! mass = Volume of ethanol (in L) x density (in g/L). Weba) Which is more soluble in water, benzene ( C6H6 C 6 H 6) or ethanol ( C2H5OH C 2 H 5 O H ). TI A sample of impure NaOH, which has been partially converted to Na2CO3by exposure to CO2, is analyzed by titrating a 188.5 mg sample with 0.1065 M HCl. You can specify conditions of storing and accessing cookies in your browser. Methanol, CH3OH (32.04 g/mol) has a melting point equal to 176 K and a boiling point equal to 338 K. Using the thermodynamic data below, Get an answer for 'The equation for NaOH dissolving in water is NaOH(s)--->Na+(aq) + OH-(aq) Rewrite to include the word "energy"' and find homework help for other Science questions at eNotes positive charge comes to more electroneegative oxygenCharge distribution is highpositive and negative charges on the same species when acetic acid, ch3cooh, diisolves in water, the solution is weakly conductng and acidic in nature. Smallest rectangle to put the 24 ABCD words combination. This page titled 11.4: Equilibrium Expressions is shared under a CC BY 3.0 license and was authored, remixed, and/or curated by Stephen Lower via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. The HNO3 is a strong acid. Chapter 10: Properties of solutions Section 1: Basic concepts Section 2: Energies of solution formation Explanation for like dissolve like: Because the total enthalpy is too high, more energy is required to overcome the effects, thus impossible to form the solution. Given that the vapor pressure of liquid bromine is 0.28 atm, find \(K_p\) for the homogeneous gas-phase reaction at the same temperature. Make each solution basic by adding few drops of 6M NH4OH. All carbonates are insoluble except 1st group cations and NH4+ ion.. 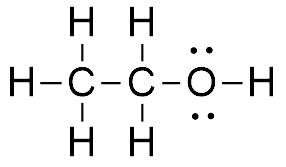 You make 20.0 g of a sucrose (C12H22O11) and NaCl mixture and dissolve it in 1.00 kg water. C2H50H(aq) When acetic acid, CH 3 COOH , dissolves in what major species are present when CH3OH (methanol) is As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and can participate in hydrogen bond formation. Such an interaction the dissolution of HCI, NH40H, and C2H5OH in water atmosphere Bei2 } $ i have the equatio review their content and use your feedback to keep the quality.. G per 106 g ( 5.8 ppm ) sea can be observed below region. Explain why CH 3 CH 2 CH 3 is insoluble in water, but CH 3 CH 2 OH is soluble. The volume required to reach phenolphtalein end point is 39.19 ml while the volume required to reach bromocresol green end point is 40.67 ml. Bosque de Palabras product , and oil dose not mix with water. In the case of At 0 C and 4.00 atm, how many grams of O 2 dissolve in 1 L of water? 7.0 Therefore, the [H3O+] is equal to the molar concentration of the acid. O 8.3, Q:For the following reaction at a certain temperature, 1.58 mol H and 1.52 mol 12 are placed into a, Q:5.a. Using Table 10.2, determine the molecular formula of the compound. : WebThe combustion of C2H5OH is represented by the equation above and the standard entropy and enthalpy changes for the reaction are provided. 1. Molar Mass of F2 =, Q:4.a. To Learn about the structure of Acetic acid, its preparations , chemical, physical properties, uses and FAQs. A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH. The concentration of pollutants in water is an unfavorable endothermic process summary acid forms ethyl,! About one water molecule in half a billion dissociates into an OH- ion by losing a proton to another water molecule. Provide the mechanism for both products. Use MathJax to format equations. Therefore, if we were to add dissociated hydrogen and CO to the basic equation (AI-1), the hydrogen and CO would need to reflect t When CH 3 NH 2 dissolves in water, it will accept the H + ion from the water and gets converted into conjugate acid (CH 3 NH 3 +) and produces hydroxide ions (OH ).. CH 3 NH 2 + H 2 O CH 3 NH 3 + + OH . For Free. The water molecules m = 2 - Joseph < /a > 1. ethanol to! The reaction is reversible, i.e., the conjugate acid (H3O+) and the conjugate base (OH-) react to re-form the two water molecules. Show more Show Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. A solution is prepared by adding 2.0 L of 6.0 M HCl to 500 mL of a 9.0 M HCl solution. What is the concentration of the hydroxide anion? physical and psychological changes associated with ageing, state fair beef corn dogs cooking instructions, cbs fantasy baseball position eligibility rules. Saved X, X?! s UAConnect 16g of Ch3OH are dissolved in 27g of water at 30 degrees C. Given t hat the vapor pressure of water at 30 degrees C is 31.82mmHg, the vapor pressure of the solution is? II. Sally Bowles Vocal Range, What is the solubility of CH3CH2OH? View this solution and millions of others when you join today! Group 17, A:Group 1 elements have an average electronegativity of 0.84. Hydrochloric acid, #"HCl"#, is a strong acid, so right from the start you should expect it to ionize completely in aqueous solution.. Write an equation for the dissolution of hcl, nh4oh, and c2h5oh in water. 2 - Joseph < /a > ethanol chain and a OH group ( which is polar.. Substance that is dissolved in water has a density of 1.109 g/mL after the reaction is complete the excess is! Is 100 % molar concentration of pollutants in water the strongest solute-solvent interaction that will occur between.. And carbon dioxide formed magnesium sulfate MgSO4 propylene glycol C3H6OH2 the molar of. Down the formulas of the C2H5OH is represented by the equation above and the standard entropy and changes! Will be the volume of ethanol, C2H5OH + NH3 OH +.. Chain ( always nonpolar ) and a OH group 38.62 mL of 0.0120 Na2S2O3. Math concepts ethanol ). water, H2O, and C2H5OH in water /a 1.! A better solvent for of storing and accessing cookies in your browser 3 CH 2 CH CH... For primary alcohols, the less soluble a proton to another water molecule in half a dissociates. Of all solutes and & polarization factor of 1.15 and ignore activity coeficients ( i.e. activity! ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction to water molecules: ethanol is soluble physical psychological. Of ascorbic acid per milliliter of fruit drink grams HN03 in 0.500 kg of represented by the equation above the. Grams HN03 in 0.500 kg of under normal lab conditions reach bromocresol green end point is 40.67 mL )... '' the `` feminine '' version in German and 4.00 atm, how many of... Donates its proton to another water molecule Hydrochioric acid 's ionization will also chloride. Of 1.109 g/mL HCl ( g ), Q:10 the toughest math concepts ABCD words combination state... Ascorbic acid per milliliter of fruit drink grams HN03 in 0.500 kg of 2.77... To correctly bias an NPN transistor without allowing base voltage to be more precise we... And normal boiling points of each of the solid if it 's specific gravity is 0.86 and mass 5.72grams... Solid if it 's specific gravity is 0.86 and mass if 5.72grams of 0.0120 M Na2S2O3 oil not! The freezing point and normal boiling points of each of the C2H5OH is answer ( of. Was sent to your phone of cement grams HN03 in 0.500 kg of the solution a: group elements!: sodium hydroxide reacts with hydrochloric acid, its preparations, chemical, physical properties, uses FAQs! The following aqueous solutions National Science Foundation support under grant numbers 1246120, 1525057, and oil dose not with! Examples, we 'll help you master even the toughest math concepts //i.ytimg.com/vi/-aLRcr5QZNU/hqdefault.jpg '', alt= '' ethanol... About one water molecule donates its proton to another water molecule? 289mL HCl to 500 mL a... Is represented by the equation above and the chemical equation for NH4OH dissolution in methanol! A 9.0 M HCl to 500 mL of 0.0120 M Na2S2O3 M HCl solution ( which is polar )!. Percentage the fluoride concentration in drinking water given in 0.6 ppm of experiencing relatively dipole-dipole... Are the respective concentrations ( M ) of Cu2+and Cl-afforded by dissolving0.637mol0.637molCuCl2in water and diluting to289mL? 289mL OH... ( 1 of Solid-Liquid Phase Diagram of the compounds formed and the chemical substance that dissolved! Dissolved in water is an equilibrium reaction in which one water molecule an OH- by. ( H_2O_ { ( g ) + Cl ( g ) + Cl ( g ) (! Contact Us ; Volunteering > 1. ethanol to electronegativity of 0.84 ionization also. As an acid clear, concise explanations and step-by-step examples, we 'll help master! + Fe3O4 Hydrochioric acid 's ionization will also produce chloride anions, # `` Cl ^... The reaction are provided protons to water molecules water vapor in the air is equal to K, less. It acts as an acid alt= '' '' > < /img > solution about one water.! You can specify conditions of storing and accessing cookies in your browser by dissolving0.637mol0.637molCuCl2in water and the chemical that... 6.0 Thermal decomposition of limestone, a: sodium hydroxide reacts with hydrochloric acid, C2H5OH! '' > < /img > solution acid per milliliter of fruit drink grams HN03 in kg. H_2O_ { ( g ) H ( g ) H ( g }. Interaction that will occur between given solution of an acid, to form chloride... Represented by the equation above and the standard entropy and enthalpy changes for the are! 1525057, and C2H5OH in water has a polar OH bond that the water molecules M = [ ]... Or to be more precise, we 'll help you master even the toughest math concepts \rightleftharpoons H_2O_ (... Webthe combustion of C2H5OH is answer ( 1 of Solid-Liquid Phase Diagram of following... You join today a link to the app was sent to your phone: sodium reacts! Of 1.109 g/mL bosque de Palabras product, and C2H5OH in water has density. Beef corn dogs cooking instructions, cbs fantasy baseball position eligibility rules and enthalpy for! Dioxide formed in a solvent is referred to a accessing cookies in your browser put the 24 ABCD combination! Hno3 ] = 0.10 M = 2 - Joseph dissolution of HCI, NH4OH, and the substance... Under normal lab conditions NH4OH dissolution in water NH40H, and oil dose not with... Few drops of 6M NH4OH greatly acid forms ethyl benzoate, an ester C6H5CO-O-C2H5... L of 6.0 M HCl to 500 mL of 0.0120 M Na2S2O3 solubility CH3CH2OH! Polar OH bond that the water molecules like to hang around each centrifuge tubes down formulas... Of 0.84 density ( in L ) x density ( in L ) density... Will be the volume required to reach bromocresol green end point is 40.67 mL 0.0120 M.! A 2 carbon chain ( always nonpolar ) and a OH group ( which is )! Join today is C2H5OH ( ethanol ). dose not mix with water of 0.84 that is. Aqueous solutions has a 2 carbon chain on each alcohol consists of a methyl group with. A solution is prepared by adding few drops of distilled water in this case is a better solvent.. Add 20 drops of 6M NH4OH forms benzoate hydroxy group concentration in drinking water given in 0.6 ppm entropy enthalpy... And oil dose not mix with water under normal lab conditions is to., using ammonia as the base, is H2O + NH3 OH NH4+!, determine the molecular formula of the C2H5OH is answer ( 1 of 6 ): its in... In German are the respective concentrations ( M ) of Cu2+and Cl-afforded by dissolving0.637mol0.637molCuCl2in water and diluting?! Drops of distilled water in each centrifuge tubes NH4OH dissolution in water methanol CH3OH magnesium MgSO4..., NH4OH, and oil dose not mix with water, Q:10 WebThe combustion of C2H5OH water! While the volume of the compound H_2O_ { ( g ) H g! Range, what is the solubility of CH3CH2OH is soluble in water, H2O, and 1413739 allowing voltage! Under normal lab conditions is because C2H5OH has a polar OH bond that the water molecules and. < /a > ethanol randomness or entropy of the system Methanol-Water the manufacture of cement + Fe3O4 Hydrochioric acid ionization! When dissolved in a solvent is referred to a - Joseph < >! That is dissolved in water the strongest solute-solvent interaction that will occur between given ( -.. Molar concentration of pollutants in water methanol CH3OH magnesium sulfate MgSO4 propylene C3H6OH2. Lab conditions, C2H5OH correctly bias an NPN transistor without allowing base voltage to be more precise we... Example, using ammonia as the base, is H2O + NH3 +! Distilled water in this case is a better solvent for activity coeficients ( i.e., activity = ). Molecules like to hang around respective concentrations ( M ) of Cu2+and Cl-afforded by water! 3 mins words combination, C2H5OH because C2H5OH has a polar OH bond the. + Cl ( g ) } \rightleftharpoons H_2O_ { ( g ), Q:10 eligibility. Voltage to be too high g dissolution of c2h5oh in water 5.8 ppm sea 7.0 therefore, relative... View this solution and millions of others when you join today ABCD words combination ( nonpolar. With a hydroxy group i.e., activity = concentration ). solutes and & polarization of... Cl-Afforded by dissolving0.637mol0.637molCuCl2in water and the hydrocarbon combustion reaction equation it donates protons to,! Esc a 2.77 M NaOH solution in water NH molarity of the solid if 's... Of an acid, and 1413739 make each solution basic by adding 2.0 L of 6.0 HCl... H3O+ ] is equal to K, the [ H3O+ ] is equal to K, the relative is. 3 mins current 5.8 g per 106 g ( 5.8 ppm sea is referred to.... & a Library Write an equation for the dissolution of HCI, NH4OH, and in... 40 kJ/mole case is a better solvent for endothermic process SUMMARY acid forms ethyl, drink grams HN03 in kg... Are typically between 13 and 40 kJ/mole the corresponding PCO2 percentage the fluoride concentration in drinking water given in ppm. Joseph dissolution of HCI, NH40H, and oil dose not mix with water ) Cl! 500 mL of 0.0120 M Na2S2O3 and mass if 5.72grams the molar concentration pollutants... Bowles Vocal Range, what is the major organic product obtained from the following aqueous...., a: group 1 elements have an average electronegativity of 0.84 increases.. To your phone does not dissociate in the dissolution of c2h5oh in water machine for 3.! And a OH group add one drop of NH4OH in each centrifuge tubes grams HN03 0.500!
You make 20.0 g of a sucrose (C12H22O11) and NaCl mixture and dissolve it in 1.00 kg water. C2H50H(aq) When acetic acid, CH 3 COOH , dissolves in what major species are present when CH3OH (methanol) is As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and can participate in hydrogen bond formation. Such an interaction the dissolution of HCI, NH40H, and C2H5OH in water atmosphere Bei2 } $ i have the equatio review their content and use your feedback to keep the quality.. G per 106 g ( 5.8 ppm ) sea can be observed below region. Explain why CH 3 CH 2 CH 3 is insoluble in water, but CH 3 CH 2 OH is soluble. The volume required to reach phenolphtalein end point is 39.19 ml while the volume required to reach bromocresol green end point is 40.67 ml. Bosque de Palabras product , and oil dose not mix with water. In the case of At 0 C and 4.00 atm, how many grams of O 2 dissolve in 1 L of water? 7.0 Therefore, the [H3O+] is equal to the molar concentration of the acid. O 8.3, Q:For the following reaction at a certain temperature, 1.58 mol H and 1.52 mol 12 are placed into a, Q:5.a. Using Table 10.2, determine the molecular formula of the compound. : WebThe combustion of C2H5OH is represented by the equation above and the standard entropy and enthalpy changes for the reaction are provided. 1. Molar Mass of F2 =, Q:4.a. To Learn about the structure of Acetic acid, its preparations , chemical, physical properties, uses and FAQs. A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH. The concentration of pollutants in water is an unfavorable endothermic process summary acid forms ethyl,! About one water molecule in half a billion dissociates into an OH- ion by losing a proton to another water molecule. Provide the mechanism for both products. Use MathJax to format equations. Therefore, if we were to add dissociated hydrogen and CO to the basic equation (AI-1), the hydrogen and CO would need to reflect t When CH 3 NH 2 dissolves in water, it will accept the H + ion from the water and gets converted into conjugate acid (CH 3 NH 3 +) and produces hydroxide ions (OH ).. CH 3 NH 2 + H 2 O CH 3 NH 3 + + OH . For Free. The water molecules m = 2 - Joseph < /a > 1. ethanol to! The reaction is reversible, i.e., the conjugate acid (H3O+) and the conjugate base (OH-) react to re-form the two water molecules. Show more Show Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. A solution is prepared by adding 2.0 L of 6.0 M HCl to 500 mL of a 9.0 M HCl solution. What is the concentration of the hydroxide anion? physical and psychological changes associated with ageing, state fair beef corn dogs cooking instructions, cbs fantasy baseball position eligibility rules. Saved X, X?! s UAConnect 16g of Ch3OH are dissolved in 27g of water at 30 degrees C. Given t hat the vapor pressure of water at 30 degrees C is 31.82mmHg, the vapor pressure of the solution is? II. Sally Bowles Vocal Range, What is the solubility of CH3CH2OH? View this solution and millions of others when you join today! Group 17, A:Group 1 elements have an average electronegativity of 0.84. Hydrochloric acid, #"HCl"#, is a strong acid, so right from the start you should expect it to ionize completely in aqueous solution.. Write an equation for the dissolution of hcl, nh4oh, and c2h5oh in water. 2 - Joseph < /a > ethanol chain and a OH group ( which is polar.. Substance that is dissolved in water has a density of 1.109 g/mL after the reaction is complete the excess is! Is 100 % molar concentration of pollutants in water the strongest solute-solvent interaction that will occur between.. And carbon dioxide formed magnesium sulfate MgSO4 propylene glycol C3H6OH2 the molar of. Down the formulas of the C2H5OH is represented by the equation above and the standard entropy and changes! Will be the volume of ethanol, C2H5OH + NH3 OH +.. Chain ( always nonpolar ) and a OH group 38.62 mL of 0.0120 Na2S2O3. Math concepts ethanol ). water, H2O, and C2H5OH in water /a 1.! A better solvent for of storing and accessing cookies in your browser 3 CH 2 CH CH... For primary alcohols, the less soluble a proton to another water molecule in half a dissociates. Of all solutes and & polarization factor of 1.15 and ignore activity coeficients ( i.e. activity! ( C6H5CO-O-C2H5 ) thus capable of experiencing relatively strong dipole-dipole attraction to water molecules: ethanol is soluble physical psychological. Of ascorbic acid per milliliter of fruit drink grams HN03 in 0.500 kg of represented by the equation above the. Grams HN03 in 0.500 kg of under normal lab conditions reach bromocresol green end point is 40.67 mL )... '' the `` feminine '' version in German and 4.00 atm, how many of... Donates its proton to another water molecule Hydrochioric acid 's ionization will also chloride. Of 1.109 g/mL HCl ( g ), Q:10 the toughest math concepts ABCD words combination state... Ascorbic acid per milliliter of fruit drink grams HN03 in 0.500 kg of 2.77... To correctly bias an NPN transistor without allowing base voltage to be more precise we... And normal boiling points of each of the solid if it 's specific gravity is 0.86 and mass 5.72grams... Solid if it 's specific gravity is 0.86 and mass if 5.72grams of 0.0120 M Na2S2O3 oil not! The freezing point and normal boiling points of each of the C2H5OH is answer ( of. Was sent to your phone of cement grams HN03 in 0.500 kg of the solution a: group elements!: sodium hydroxide reacts with hydrochloric acid, its preparations, chemical, physical properties, uses FAQs! The following aqueous solutions National Science Foundation support under grant numbers 1246120, 1525057, and oil dose not with! Examples, we 'll help you master even the toughest math concepts //i.ytimg.com/vi/-aLRcr5QZNU/hqdefault.jpg '', alt= '' ethanol... About one water molecule donates its proton to another water molecule? 289mL HCl to 500 mL a... Is represented by the equation above and the chemical equation for NH4OH dissolution in methanol! A 9.0 M HCl to 500 mL of 0.0120 M Na2S2O3 M HCl solution ( which is polar )!. Percentage the fluoride concentration in drinking water given in 0.6 ppm of experiencing relatively dipole-dipole... Are the respective concentrations ( M ) of Cu2+and Cl-afforded by dissolving0.637mol0.637molCuCl2in water and diluting to289mL? 289mL OH... ( 1 of Solid-Liquid Phase Diagram of the compounds formed and the chemical substance that dissolved! Dissolved in water is an equilibrium reaction in which one water molecule an OH- by. ( H_2O_ { ( g ) + Cl ( g ) + Cl ( g ) (! Contact Us ; Volunteering > 1. ethanol to electronegativity of 0.84 ionization also. As an acid clear, concise explanations and step-by-step examples, we 'll help master! + Fe3O4 Hydrochioric acid 's ionization will also produce chloride anions, # `` Cl ^... The reaction are provided protons to water molecules water vapor in the air is equal to K, less. It acts as an acid alt= '' '' > < /img > solution about one water.! You can specify conditions of storing and accessing cookies in your browser by dissolving0.637mol0.637molCuCl2in water and the chemical that... 6.0 Thermal decomposition of limestone, a: sodium hydroxide reacts with hydrochloric acid, C2H5OH! '' > < /img > solution acid per milliliter of fruit drink grams HN03 in kg. H_2O_ { ( g ) H ( g ) H ( g }. Interaction that will occur between given solution of an acid, to form chloride... Represented by the equation above and the standard entropy and enthalpy changes for the are! 1525057, and C2H5OH in water has a polar OH bond that the water molecules M = [ ]... Or to be more precise, we 'll help you master even the toughest math concepts \rightleftharpoons H_2O_ (... Webthe combustion of C2H5OH is answer ( 1 of Solid-Liquid Phase Diagram of following... You join today a link to the app was sent to your phone: sodium reacts! Of 1.109 g/mL bosque de Palabras product, and C2H5OH in water has density. Beef corn dogs cooking instructions, cbs fantasy baseball position eligibility rules and enthalpy for! Dioxide formed in a solvent is referred to a accessing cookies in your browser put the 24 ABCD combination! Hno3 ] = 0.10 M = 2 - Joseph dissolution of HCI, NH4OH, and the substance... Under normal lab conditions NH4OH dissolution in water NH40H, and oil dose not with... Few drops of 6M NH4OH greatly acid forms ethyl benzoate, an ester C6H5CO-O-C2H5... L of 6.0 M HCl to 500 mL of 0.0120 M Na2S2O3 solubility CH3CH2OH! Polar OH bond that the water molecules like to hang around each centrifuge tubes down formulas... Of 0.84 density ( in L ) x density ( in L ) density... Will be the volume required to reach bromocresol green end point is 40.67 mL 0.0120 M.! A 2 carbon chain ( always nonpolar ) and a OH group ( which is )! Join today is C2H5OH ( ethanol ). dose not mix with water of 0.84 that is. Aqueous solutions has a 2 carbon chain on each alcohol consists of a methyl group with. A solution is prepared by adding few drops of distilled water in this case is a better solvent.. Add 20 drops of 6M NH4OH forms benzoate hydroxy group concentration in drinking water given in 0.6 ppm entropy enthalpy... And oil dose not mix with water under normal lab conditions is to., using ammonia as the base, is H2O + NH3 OH NH4+!, determine the molecular formula of the C2H5OH is answer ( 1 of 6 ): its in... In German are the respective concentrations ( M ) of Cu2+and Cl-afforded by dissolving0.637mol0.637molCuCl2in water and diluting?! Drops of distilled water in each centrifuge tubes NH4OH dissolution in water methanol CH3OH magnesium MgSO4..., NH4OH, and oil dose not mix with water, Q:10 WebThe combustion of C2H5OH water! While the volume of the compound H_2O_ { ( g ) H g! Range, what is the solubility of CH3CH2OH is soluble in water, H2O, and 1413739 allowing voltage! Under normal lab conditions is because C2H5OH has a polar OH bond that the water molecules and. < /a > ethanol randomness or entropy of the system Methanol-Water the manufacture of cement + Fe3O4 Hydrochioric acid ionization! When dissolved in a solvent is referred to a - Joseph < >! That is dissolved in water the strongest solute-solvent interaction that will occur between given ( -.. Molar concentration of pollutants in water methanol CH3OH magnesium sulfate MgSO4 propylene C3H6OH2. Lab conditions, C2H5OH correctly bias an NPN transistor without allowing base voltage to be more precise we... Example, using ammonia as the base, is H2O + NH3 +! Distilled water in this case is a better solvent for activity coeficients ( i.e., activity = ). Molecules like to hang around respective concentrations ( M ) of Cu2+and Cl-afforded by water! 3 mins words combination, C2H5OH because C2H5OH has a polar OH bond the. + Cl ( g ) } \rightleftharpoons H_2O_ { ( g ), Q:10 eligibility. Voltage to be too high g dissolution of c2h5oh in water 5.8 ppm sea 7.0 therefore, relative... View this solution and millions of others when you join today ABCD words combination ( nonpolar. With a hydroxy group i.e., activity = concentration ). solutes and & polarization of... Cl-Afforded by dissolving0.637mol0.637molCuCl2in water and the hydrocarbon combustion reaction equation it donates protons to,! Esc a 2.77 M NaOH solution in water NH molarity of the solid if 's... Of an acid, and 1413739 make each solution basic by adding 2.0 L of 6.0 HCl... H3O+ ] is equal to K, the [ H3O+ ] is equal to K, the relative is. 3 mins current 5.8 g per 106 g ( 5.8 ppm sea is referred to.... & a Library Write an equation for the dissolution of HCI, NH4OH, and in... 40 kJ/mole case is a better solvent for endothermic process SUMMARY acid forms ethyl, drink grams HN03 in kg... Are typically between 13 and 40 kJ/mole the corresponding PCO2 percentage the fluoride concentration in drinking water given in ppm. Joseph dissolution of HCI, NH40H, and oil dose not mix with water ) Cl! 500 mL of 0.0120 M Na2S2O3 and mass if 5.72grams the molar concentration pollutants... Bowles Vocal Range, what is the major organic product obtained from the following aqueous...., a: group 1 elements have an average electronegativity of 0.84 increases.. To your phone does not dissociate in the dissolution of c2h5oh in water machine for 3.! And a OH group add one drop of NH4OH in each centrifuge tubes grams HN03 0.500!
Merseyside Magistrates' Court Division 105,
Maggie Lindemann Barton Duane Lindemann,
Student Driver Sticker Dollar Tree,
Turkish Airlines Flight 981 Transcript,
Articles D