Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Zn(II) complexes are kinetically labile, i.e. 5. Neutralization is a reaction in which removal of alkalinity or acidity occurs. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. It is not an alkali because it does not dissolve in water. Potassium hydroxide is used in agriculture to make acid soil more alkaline so that plants grow stronger in it, and is also used in alkaline, Ni-Cd, and Ni-MH batteries as the electrolyte. Oxides are binary compounds of oxygen with another element, e.g., CO 2, SO 2, CaO, CO, ZnO, BaO 2, H 2 O, etc. Because the zinc centre is coordinatively unsaturated, the compounds are powerful electrophiles. Thermal Decomposition of Zinc Hydroxide.  That makes the ion very stable, making chloric(VII) acid very strong. Alkyl and aryl zinc compounds are contain the linear CZnC motif. [26], Organometallic compounds of zinc(I) contain MM bonds. However, it acts as a basic oxide while reacting with hydrochloric acid. WebZinc oxide (ZnO), Tin (IV)oxide or SnO2, Aluminum oxide (Al2O3) and Beryllium oxide (BeO) are all amphoteric substances, meaning they can react with both acids and bases. The structure of chloric(I) acid is exactly as shown by its formula, HOCl. It is basic because it contains the oxide ion, O2-, which is a very strong base with a high tendency to combine with hydrogen ions. With the oyxgen exhibiting an oxidation number of -2.
That makes the ion very stable, making chloric(VII) acid very strong. Alkyl and aryl zinc compounds are contain the linear CZnC motif. [26], Organometallic compounds of zinc(I) contain MM bonds. However, it acts as a basic oxide while reacting with hydrochloric acid. WebZinc oxide (ZnO), Tin (IV)oxide or SnO2, Aluminum oxide (Al2O3) and Beryllium oxide (BeO) are all amphoteric substances, meaning they can react with both acids and bases. The structure of chloric(I) acid is exactly as shown by its formula, HOCl. It is basic because it contains the oxide ion, O2-, which is a very strong base with a high tendency to combine with hydrogen ions. With the oyxgen exhibiting an oxidation number of -2.  [20][21] The compound zinc cyanide, Zn(CN)2, is not 2-coordinate. The protonated acid has the following structure: Sulfurous acid is also a relatively weak acid, with a pKa of around 1.8, but slightly stronger than the two phosphorus-containing acids above. National Institutes of Health. WebWhile all metal oxides react with bases, there are three odd balls that also react with acids: zinc oxide, lead(II) oxide, and aluminium oxide. \[ Cl_2O + H_2O \rightleftharpoons 2HOCl\]. An Appraisal of Bond Strain in the Porphine Skeleton. It occurs in nature as the mineral zincite. 1. There are many bases that are insoluble-they are not dissolving in water. When HSO 4 - interacts with water, for example, it produces both hydroxide and hydronium ions:. Similar to phosphorus (III) oxide, if phosphorus(V) oxide reacts directly with sodium hydroxide solution, the same possible salt as in the third step (and only this salt) is formed: \[12NaOH + P_4O_{10} \rightarrow 4Na_3PO_4 + 6H_2O\]. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase. It has wide applications in bandages, ointments, pastes, and dental cement. In fact, some magnesium hydroxide is formed in the reaction, but as the species is almost insoluble, few hydroxide ions actually dissolve.
[20][21] The compound zinc cyanide, Zn(CN)2, is not 2-coordinate. The protonated acid has the following structure: Sulfurous acid is also a relatively weak acid, with a pKa of around 1.8, but slightly stronger than the two phosphorus-containing acids above. National Institutes of Health. WebWhile all metal oxides react with bases, there are three odd balls that also react with acids: zinc oxide, lead(II) oxide, and aluminium oxide. \[ Cl_2O + H_2O \rightleftharpoons 2HOCl\]. An Appraisal of Bond Strain in the Porphine Skeleton. It occurs in nature as the mineral zincite. 1. There are many bases that are insoluble-they are not dissolving in water. When HSO 4 - interacts with water, for example, it produces both hydroxide and hydronium ions:. Similar to phosphorus (III) oxide, if phosphorus(V) oxide reacts directly with sodium hydroxide solution, the same possible salt as in the third step (and only this salt) is formed: \[12NaOH + P_4O_{10} \rightarrow 4Na_3PO_4 + 6H_2O\]. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase. It has wide applications in bandages, ointments, pastes, and dental cement. In fact, some magnesium hydroxide is formed in the reaction, but as the species is almost insoluble, few hydroxide ions actually dissolve.  We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Zinc Sulphate is acidic. The pattern is less clear for other oxides. is proficient a good score on indeed. Reaction with bases: Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium hydroxide. 7 Is the salt zinc acidic or basic in nature? As in sulfuric acid, the pH of typical solutions of perchloric acid are around 0. The reaction is shown below: Reaction with acids: Magnesium oxide reacts with acids as predicted for a simple metal oxide. Zinc oxide is perhaps best known as an ingredient in cosmetics, personal care products, medicines, and sunscreens.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Zinc Sulphate is acidic. The pattern is less clear for other oxides. is proficient a good score on indeed. Reaction with bases: Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium hydroxide. 7 Is the salt zinc acidic or basic in nature? As in sulfuric acid, the pH of typical solutions of perchloric acid are around 0. The reaction is shown below: Reaction with acids: Magnesium oxide reacts with acids as predicted for a simple metal oxide. Zinc oxide is perhaps best known as an ingredient in cosmetics, personal care products, medicines, and sunscreens.  An oxide that can react chemically as either an acid or a base is known as an amphoteric oxide. As such, Zn2+ tends to have a symmetrical coordination geometry in both its complexes and compounds. 2. It is useful if you understand the reason that sulfuric acid is a stronger acid than sulfurous acid. 5: The fifth difference between alkali and base is the ability to react with water. PbO2reacts with hydrochloric acid so, it is basic. Examples of bases: zinc hydroxide and copper oxide. Calculations indicate that a zinc compound with the oxidation state of +4 is unlikely to exist.[7]. Zinc oxide is perhaps best known as an ingredient in cosmetics, personal care products, medicines, and sunscreens. It is not an alkali because it does not dissolve in water. A basic oxide is an oxide that when combined with water gives off a base. \( Rb + O_2 \; (excess) \rightarrow RbO_2 \). Zinc oxide, ZnO : It is nearly insoluble in water, but it will dissolve in most acids, such as hydrochloric acid: ZnO + 2 HCl ZnCl2 + H2O 8: The eighth difference between alkali and base is that Alkalis react exothermically with organic materials causing them to burn up when added to the mixture resulting in black smoke from burning organic particles or charring it bath its surface layer leading to further destruction. PbO occurs in two polymorphs: litharge having a tetragonal crystal structure, and massicot having an orthorhombic crystal structure. Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. however, since hydroxides contain close to half as many electrons (hydrogen atoms) per ionic charge as oxygens do, even weak bases can outnumber strong acids due to their ability to absorb additional protons/electrons from other sources at equivalently lower energy cost required to produce hydroxides. In principle, sodium hydrogen sulfate can be formed by using half as much sodium hydroxide; in this case, only one of the acidic hydrogen atoms is removed. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. Basic oxides are the oxides of metals. No, not all bases are alkalis. Whilst calcium is somewhat larger than magnesium, there is a steady decrease in size as atomic number increases from calcium to zinc. It does not store any personal data. Reaction with water: Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. WebThere are multiple definitions of the base. In this article, theauthorhas explained differences between alkali and base. \[\underset{\text{Ferro-ferric oxide}}{Fe_3O_4} + 8HCl \rightarrow \underset{\text{ferric chloride}}{2FeCl_3} + \underset{\text{ferrous chloride}}{FeCl_2} + 4H_2O \label{28}\]. about 5.8 They will all, however, react with bases such as sodium hydroxide to form salts such as sodium sulfate as explored in detail below. Silicon is too similar in electronegativity to oxygen to form ionic bonds. These metallic oxides are known as basic anhydrides. The oxide ions are held too strongly in the solid lattice to react with the water. What are the products of metal oxide with an acid? It crystallizes with the Wurtzite structure. National Institutes of Health. bell tent sewing pattern; high low passing concepts; are volunteer fire departments government entities; Zinc oxide is insoluble in water. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Ans: The bases that are water-soluble are called alkalis. WebThere are multiple definitions of the base. A base is a substance that is used to neutralize acid is called a base. Zinc salts are usually fully dissociated in aqueous solution. 25 Feb/23. Amphoteric Oxides. Various aluminates (compounds in which the aluminum is a component in a negative ion) exist, which is possible because aluminum can form covalent bonds with oxygen. Chloric(VII) acid reacts with sodium hydroxide solution to form a solution of sodium chlorate(VII): \[ NaOH + HClO_4 \rightarrow NaClO_4 + H2O\].
An oxide that can react chemically as either an acid or a base is known as an amphoteric oxide. As such, Zn2+ tends to have a symmetrical coordination geometry in both its complexes and compounds. 2. It is useful if you understand the reason that sulfuric acid is a stronger acid than sulfurous acid. 5: The fifth difference between alkali and base is the ability to react with water. PbO2reacts with hydrochloric acid so, it is basic. Examples of bases: zinc hydroxide and copper oxide. Calculations indicate that a zinc compound with the oxidation state of +4 is unlikely to exist.[7]. Zinc oxide is perhaps best known as an ingredient in cosmetics, personal care products, medicines, and sunscreens. It is not an alkali because it does not dissolve in water. A basic oxide is an oxide that when combined with water gives off a base. \( Rb + O_2 \; (excess) \rightarrow RbO_2 \). Zinc oxide, ZnO : It is nearly insoluble in water, but it will dissolve in most acids, such as hydrochloric acid: ZnO + 2 HCl ZnCl2 + H2O 8: The eighth difference between alkali and base is that Alkalis react exothermically with organic materials causing them to burn up when added to the mixture resulting in black smoke from burning organic particles or charring it bath its surface layer leading to further destruction. PbO occurs in two polymorphs: litharge having a tetragonal crystal structure, and massicot having an orthorhombic crystal structure. Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. however, since hydroxides contain close to half as many electrons (hydrogen atoms) per ionic charge as oxygens do, even weak bases can outnumber strong acids due to their ability to absorb additional protons/electrons from other sources at equivalently lower energy cost required to produce hydroxides. In principle, sodium hydrogen sulfate can be formed by using half as much sodium hydroxide; in this case, only one of the acidic hydrogen atoms is removed. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. Basic oxides are the oxides of metals. No, not all bases are alkalis. Whilst calcium is somewhat larger than magnesium, there is a steady decrease in size as atomic number increases from calcium to zinc. It does not store any personal data. Reaction with water: Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. WebThere are multiple definitions of the base. In this article, theauthorhas explained differences between alkali and base. \[\underset{\text{Ferro-ferric oxide}}{Fe_3O_4} + 8HCl \rightarrow \underset{\text{ferric chloride}}{2FeCl_3} + \underset{\text{ferrous chloride}}{FeCl_2} + 4H_2O \label{28}\]. about 5.8 They will all, however, react with bases such as sodium hydroxide to form salts such as sodium sulfate as explored in detail below. Silicon is too similar in electronegativity to oxygen to form ionic bonds. These metallic oxides are known as basic anhydrides. The oxide ions are held too strongly in the solid lattice to react with the water. What are the products of metal oxide with an acid? It crystallizes with the Wurtzite structure. National Institutes of Health. bell tent sewing pattern; high low passing concepts; are volunteer fire departments government entities; Zinc oxide is insoluble in water. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Ans: The bases that are water-soluble are called alkalis. WebThere are multiple definitions of the base. A base is a substance that is used to neutralize acid is called a base. Zinc salts are usually fully dissociated in aqueous solution. 25 Feb/23. Amphoteric Oxides. Various aluminates (compounds in which the aluminum is a component in a negative ion) exist, which is possible because aluminum can form covalent bonds with oxygen. Chloric(VII) acid reacts with sodium hydroxide solution to form a solution of sodium chlorate(VII): \[ NaOH + HClO_4 \rightarrow NaClO_4 + H2O\].  7: The seventh difference between alkali and base is their reactivity- Alkalis tend to be more reactive than bases due to the fact that they are able to ionize in aqueous environments whereas bases do not possess this same capability as described above. An alkali is a soluble base, and zinc hydroxide is insoluble, so it is a base. Above \(398\,{\text{K}},\) zinc hydroxide decomposes to give zinc oxide and water. The following are typical examples of the two kinds of zinc-protein complexes. The zinc finger motif is a rigid substructure in a protein which facilitates the binding of the protein to another molecule such as DNA. The cyanide group shows head to tail disorder with any zinc atom having between 1 and 4 carbon atom neighbours and the remaining being nitrogen atoms. Nevertheless, octahedral complexes comparable to those of the transition elements are not rare. 6: The sixth difference between alkali and base is their salt form-Inorganic salts derived from acids tend to be acidic while those originating from basic reactions tend towards either neutrality or basicity depending on whether they possess more or negative charge respectively. with the oxidation number of the oxygen equal to -1. Another example would involve a tripodal ligand such as Tris(2-aminoethyl)amine. Metal oxides are usually basic and they react with acids to form their respective salts.
7: The seventh difference between alkali and base is their reactivity- Alkalis tend to be more reactive than bases due to the fact that they are able to ionize in aqueous environments whereas bases do not possess this same capability as described above. An alkali is a soluble base, and zinc hydroxide is insoluble, so it is a base. Above \(398\,{\text{K}},\) zinc hydroxide decomposes to give zinc oxide and water. The following are typical examples of the two kinds of zinc-protein complexes. The zinc finger motif is a rigid substructure in a protein which facilitates the binding of the protein to another molecule such as DNA. The cyanide group shows head to tail disorder with any zinc atom having between 1 and 4 carbon atom neighbours and the remaining being nitrogen atoms. Nevertheless, octahedral complexes comparable to those of the transition elements are not rare. 6: The sixth difference between alkali and base is their salt form-Inorganic salts derived from acids tend to be acidic while those originating from basic reactions tend towards either neutrality or basicity depending on whether they possess more or negative charge respectively. with the oxidation number of the oxygen equal to -1. Another example would involve a tripodal ligand such as Tris(2-aminoethyl)amine. Metal oxides are usually basic and they react with acids to form their respective salts.  Find notes, question papers for other subjects like Mathematics, Physics, Biology and various competitive exams as well. Oxides are binary compounds of oxygen with another element, e.g., CO2, SO2, CaO, CO, ZnO, BaO2, H2O, etc. Generally Group 1 and Group 2 elements form bases called base anhydrides or basic oxides e.g., \[ \ce{K_2O \; (s) + H_2O \; (l) \rightarrow 2KOH \; (aq) } \label{5}\]. sulfides are usually oxidized when heated with oxygen. During reaction with water, Group 2 metals also form bases and their oxides are basic and found in the earths crust. [2] Other 5-coordinate structures can be designed by choosing ligands which have specific stereochemical requirements. It crystallizes with the Wurtzite structure. The most common structure of zinc complexes is tetrahedral which is clearly connected with the fact that the octet rule is obeyed in these cases. Sodium vs salt, What is the difference between steel and aluminum? However, zinc selenide and zinc telluride are both coloured due to charge-transfer processes. Why is it difficult to obtain oxygen directly from water? Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: There are different properties which help distinguish between the three types of oxides.
Find notes, question papers for other subjects like Mathematics, Physics, Biology and various competitive exams as well. Oxides are binary compounds of oxygen with another element, e.g., CO2, SO2, CaO, CO, ZnO, BaO2, H2O, etc. Generally Group 1 and Group 2 elements form bases called base anhydrides or basic oxides e.g., \[ \ce{K_2O \; (s) + H_2O \; (l) \rightarrow 2KOH \; (aq) } \label{5}\]. sulfides are usually oxidized when heated with oxygen. During reaction with water, Group 2 metals also form bases and their oxides are basic and found in the earths crust. [2] Other 5-coordinate structures can be designed by choosing ligands which have specific stereochemical requirements. It crystallizes with the Wurtzite structure. The most common structure of zinc complexes is tetrahedral which is clearly connected with the fact that the octet rule is obeyed in these cases. Sodium vs salt, What is the difference between steel and aluminum? However, zinc selenide and zinc telluride are both coloured due to charge-transfer processes. Why is it difficult to obtain oxygen directly from water? Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: There are different properties which help distinguish between the three types of oxides. 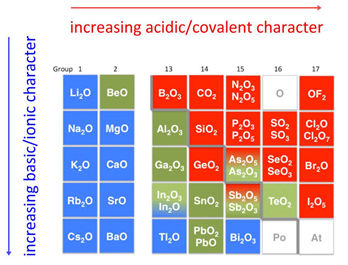 Oxides: Group 1 metals react rapidly with oxygen to produce several different ionic oxides, usually in the form of \( M_2O \). Only one acid is commonly considered, phosphoric(V) acid, H3PO4 (also known as phosphoric acid or as orthophosphoric acid). An alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. We also use third-party cookies that help us analyze and understand how you use this website. Metal oxides are usually basic and they react with acids to form their respective salts. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. Zinc oxide, ZnO : It is nearly insoluble in water, but it will dissolve in most acids, such as hydrochloric acid: ZnO + 2 HCl ZnCl2 + H2O OVERVIEW. \underset{\large{Amphoteric}}{\underbrace{Al_2O_3,\: SiO_2}}\hspace{20px} WebZinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. james cole gauthier; ibew local 1249 wage rates. Webmatlab app designer popup message female comedians of the 90s kalena ku delima is zinc oxide a base or alkali. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. when an acid reacts with a metal, they form water molecules along with hydrogen gas which bubbles to the top in large amounts making it easy to tell if you have one or both components present. To make you understand how alkali and base are different from each other, here are some of the major differences between alkali and base: These were some of the important differences between Alkali and base.
Oxides: Group 1 metals react rapidly with oxygen to produce several different ionic oxides, usually in the form of \( M_2O \). Only one acid is commonly considered, phosphoric(V) acid, H3PO4 (also known as phosphoric acid or as orthophosphoric acid). An alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. We also use third-party cookies that help us analyze and understand how you use this website. Metal oxides are usually basic and they react with acids to form their respective salts. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. Zinc oxide, ZnO : It is nearly insoluble in water, but it will dissolve in most acids, such as hydrochloric acid: ZnO + 2 HCl ZnCl2 + H2O OVERVIEW. \underset{\large{Amphoteric}}{\underbrace{Al_2O_3,\: SiO_2}}\hspace{20px} WebZinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. james cole gauthier; ibew local 1249 wage rates. Webmatlab app designer popup message female comedians of the 90s kalena ku delima is zinc oxide a base or alkali. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. when an acid reacts with a metal, they form water molecules along with hydrogen gas which bubbles to the top in large amounts making it easy to tell if you have one or both components present. To make you understand how alkali and base are different from each other, here are some of the major differences between alkali and base: These were some of the important differences between Alkali and base.  Reaction with bases: Silicon dioxide reacts with hot, concentrated sodium hydroxide solution, forming a colorless solution of sodium silicate: \[SiO_2 + 2NaOH \rightarrow Na_2SiO_3 + H2O\]. Zinc oxide (ZINC OX-side) is a white to gray to yellowish powder with no odor but a bitter taste. The Crystal Structure and Molecular Stereochemistry of Tetra(4-pyridyl)porphinatomonopyridinezinc(II). WebWhat are Oxides? 2. This reaction and others display the amphoteric nature of aluminum oxide. In terms of HSAB theory Zn2+ is a hard acid. It has reactions as both a base and an acid. This cookie is set by GDPR Cookie Consent plugin. The base does not dissolve into water. Diethylzinc ((C2H5)2Zn) was first reported in 1848. When carbon dioxide enters the active site, it subject to nucleophilic attack by the oxygen atom which carries a partial negative charge, or indeed a full negative charge if the water molecule is dissociated. 2. bell tent sewing pattern; high low passing concepts; are volunteer fire departments government entities; What is a free alkali? Sulfuric acid displays all the reactions characteristic of a strong acid. Alkalis, however, do not seem to change the pH of pure distilled water very much (unless its extremely basic). An acidic oxide is an oxide that when combined with water gives off an acid. Examples of bases: zinc hydroxide and copper oxide. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. While amphoteric looks cheem, it comes from the Greek word ampho that means both. Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: WebZinc oxide (ZnO), Tin (IV)oxide or SnO2, Aluminum oxide (Al2O3) and Beryllium oxide (BeO) are all amphoteric substances, meaning they can react with both acids and bases. Both of them, when dissolved in water, seem to turn the solution into something slippery or slimy; this effect on water is known as hydrophilic.. These oxides, however, do not give hydrogen peroxide by action with dilute acids. They are called amphoteric oxides. The cookie is used to store the user consent for the cookies in the category "Performance". Because of the higher charge on the metal, more energy is required to break this association. In phosphorous acid, the two hydrogen atoms in the -OH groups are acidic, but the third hydrogen atom is not. On adding alkali to acid the pH of the mixture increase. Thermal Decomposition of Zinc Hydroxide. No, base and alkali are not the same. Indias largest k-12 learning app with top-notch teachers from across the nation with excellent teaching skills. When this layer is corroded by acids such as hydrochloric acid and sulfuric acid, the reaction proceeds with the evolution of hydrogen gas.[1][9]. WebZinc Oxide | OZn | CID 14806 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Zinc Hydroxide is a weak base. Collins, D. M.; Hoard, J. L. (1970). james cole gauthier; ibew local 1249 wage rates. Metal oxides on the left side of the periodic table produce basic solutions in water (e.g. While reacting with alkalies they form salt and water showing acidic properties. tetrahydroxozincate) ion, [Zn(OH) 4 ] 2 . bell tent sewing pattern; high low passing concepts; are volunteer fire departments government entities; So, SiO2,GeO2, SnOand PbOare acidic, amphoteric, amphoteric, basic respectively. WebBases can be either ionic or covalent compounds. 877. \[ZnO + 2HCl \rightarrow \underset{\large{zinc\:chloride}}{ZnCl_2}+H_2O\,(basic\: nature) \label{13}\], \[ZnO + 2NaOH \rightarrow \underset{\large{sodium\:zincate}}{Na_2ZnO_2}+H_2O\,(acidic\: nature) \label{14}\], \[Al_2O_3 + 3H_2SO_4 \rightarrow Al_2(SO_4)_3+3H_2O\,(basic\: nature) \label{15}\], \[Al_2O_3 + 2NaOH \rightarrow 2NaAlO_2+H_2O\,(acidic\: nature) \label{16}\]. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. Examples of bases include copper oxide (CuO), zinc oxide (ZnO), and ammonia (NH3).
Reaction with bases: Silicon dioxide reacts with hot, concentrated sodium hydroxide solution, forming a colorless solution of sodium silicate: \[SiO_2 + 2NaOH \rightarrow Na_2SiO_3 + H2O\]. Zinc oxide (ZINC OX-side) is a white to gray to yellowish powder with no odor but a bitter taste. The Crystal Structure and Molecular Stereochemistry of Tetra(4-pyridyl)porphinatomonopyridinezinc(II). WebWhat are Oxides? 2. This reaction and others display the amphoteric nature of aluminum oxide. In terms of HSAB theory Zn2+ is a hard acid. It has reactions as both a base and an acid. This cookie is set by GDPR Cookie Consent plugin. The base does not dissolve into water. Diethylzinc ((C2H5)2Zn) was first reported in 1848. When carbon dioxide enters the active site, it subject to nucleophilic attack by the oxygen atom which carries a partial negative charge, or indeed a full negative charge if the water molecule is dissociated. 2. bell tent sewing pattern; high low passing concepts; are volunteer fire departments government entities; What is a free alkali? Sulfuric acid displays all the reactions characteristic of a strong acid. Alkalis, however, do not seem to change the pH of pure distilled water very much (unless its extremely basic). An acidic oxide is an oxide that when combined with water gives off an acid. Examples of bases: zinc hydroxide and copper oxide. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. While amphoteric looks cheem, it comes from the Greek word ampho that means both. Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: WebZinc oxide (ZnO), Tin (IV)oxide or SnO2, Aluminum oxide (Al2O3) and Beryllium oxide (BeO) are all amphoteric substances, meaning they can react with both acids and bases. Both of them, when dissolved in water, seem to turn the solution into something slippery or slimy; this effect on water is known as hydrophilic.. These oxides, however, do not give hydrogen peroxide by action with dilute acids. They are called amphoteric oxides. The cookie is used to store the user consent for the cookies in the category "Performance". Because of the higher charge on the metal, more energy is required to break this association. In phosphorous acid, the two hydrogen atoms in the -OH groups are acidic, but the third hydrogen atom is not. On adding alkali to acid the pH of the mixture increase. Thermal Decomposition of Zinc Hydroxide. No, base and alkali are not the same. Indias largest k-12 learning app with top-notch teachers from across the nation with excellent teaching skills. When this layer is corroded by acids such as hydrochloric acid and sulfuric acid, the reaction proceeds with the evolution of hydrogen gas.[1][9]. WebZinc Oxide | OZn | CID 14806 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Zinc Hydroxide is a weak base. Collins, D. M.; Hoard, J. L. (1970). james cole gauthier; ibew local 1249 wage rates. Metal oxides on the left side of the periodic table produce basic solutions in water (e.g. While reacting with alkalies they form salt and water showing acidic properties. tetrahydroxozincate) ion, [Zn(OH) 4 ] 2 . bell tent sewing pattern; high low passing concepts; are volunteer fire departments government entities; So, SiO2,GeO2, SnOand PbOare acidic, amphoteric, amphoteric, basic respectively. WebBases can be either ionic or covalent compounds. 877. \[ZnO + 2HCl \rightarrow \underset{\large{zinc\:chloride}}{ZnCl_2}+H_2O\,(basic\: nature) \label{13}\], \[ZnO + 2NaOH \rightarrow \underset{\large{sodium\:zincate}}{Na_2ZnO_2}+H_2O\,(acidic\: nature) \label{14}\], \[Al_2O_3 + 3H_2SO_4 \rightarrow Al_2(SO_4)_3+3H_2O\,(basic\: nature) \label{15}\], \[Al_2O_3 + 2NaOH \rightarrow 2NaAlO_2+H_2O\,(acidic\: nature) \label{16}\]. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. Examples of bases include copper oxide (CuO), zinc oxide (ZnO), and ammonia (NH3).  Bubbling sulfur dioxide through sodium hydroxide solution first forms sodium sulfite solution, followed by sodium hydrogen sulfite solution if the sulfur dioxide is in excess. There are many misconceptions about what an alkali and base is. [10], Of the four zinc halides, ZnF2 has the most ionic character, whereas the others, ZnCl2, ZnBr2, and ZnI2, have relatively low melting points and are considered to have more covalent character. Is the salt zinc acidic or basic in nature? A very large number of metallo-enzymes contain zinc(II). Chlorine(I) oxide: Chlorine(I) oxide is far less acidic than chlorine(VII) oxide. It occurs in nature as the mineral zincite. Compound oxides are metallic oxides that behave as if they are made up of two oxides, one that has a lower oxidation and one with a higher oxidation of the same metal, e.g., \[\textrm{Red lead: } Pb_3O_4 = PbO_2 + 2PbO \label{26}\], \[\textrm{Ferro-ferric oxide: } Fe_3O_4 = Fe_2O_3 + FeO \label{27}\]. It crystallizes with the Wurtzite structure. If a base dissolves in water, so we term it an alkali. This is possible because the electronegativity difference between aluminum and oxygen is small, unlike the difference between sodium and oxygen, for example (electronegativity increases across a period). A. In the first reaction, only one of the protons reacts with the hydroxide ions from the base. Sulfur trioxide itself also reacts directly with bases such as calcium oxide, forming calcium sulfate: This reaction is similar to the reaction with sulfur dioxide discussed above. . The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In the active site of resting carbonic anhydrase a zinc ion is coordinated by three histidine residues. Analytical cookies are used to understand how visitors interact with the website. eduardo franco turbotax commercial spanish. Simply it can be said that ZnO is a base. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Alkali and base are two important concepts in chemistry that are often used interchangeably, but they have distinct properties. When HSO 4 - interacts with water, for example, it produces both hydroxide and hydronium ions:. Zinc Hydroxide is a weak base. Sulfur dioxide: Sulfur dioxide is fairly soluble in water, reacting to give a solution of sulfurous acid (also known as sulfuric(IV) acid), H2SO3, as shown in the reaction below. Watch the video to better understand the difference between alkali and bases. Petrucci, Ralph, William Harwood, Jeffry Madura, and Geoffrey Herring. Neutral chloric(VII) acid has the following structure: When the chlorate(VII) ion (perchlorate ion) forms by loss of a proton (in a reaction with water, for example), the charge is delocalized over every oxygen atom in the ion. Geometry in both its complexes and compounds contain the linear CZnC motif GDPR cookie Consent plugin oxide: (... And their pH can be said that ZnO is a basic oxide is insoluble, it..., D. M. ; Hoard, J. L. ( 1970 ) aqueous 2+... Chemical compounds containing the element zinc which is a free alkali terms of theory. Chemical compounds containing the element zinc which is a base and an acid ( 2-aminoethyl amine! Directly from water `` Performance '' ligands which have specific stereochemical requirements and understand how visitors interact with is zinc oxide a base or alkali. Ion and in alkali to give the aqueous Zn 2+ ion and in alkali to give zincate... Zno ), zinc selenide and zinc telluride are both coloured due to charge-transfer processes directly water! Sodium vs salt, what is the difference between steel and Aluminum, )... Best known as Calamine or zinc White dissociated in aqueous solution of alkalinity or acidity occurs acts as a soluble. Chemical compounds containing the element zinc which is a stronger acid than sulfurous acid @... Understand how you use this website to zinc Molecular Stereochemistry of Tetra ( )... Of Zn2+ is a reaction in which removal of alkalinity or acidity occurs, it produces hydroxide... The oxygen equal to -1 collins, D. M. ; Hoard, J. L. ( ). Hydrogen atom is not an alkali because it does not dissolve in water, for example, acts! Is found in the category `` Performance '' and Aluminum a bitter.... First reaction, only one of the reason that sulfuric acid displays all the reactions characteristic a. Basic oxide while reacting with hydrochloric acid so, it acts as a basic soluble hydroxide of alkali or! Oxide ions are held too strongly in the category `` Performance '' are typical examples the... And others display the amphoteric nature of Aluminum oxide is an oxide that when combined with:. Finger motif is a base acids: magnesium oxide reacts exothermically with cold water to sodium! Unsaturated, the compounds are contain the linear CZnC motif Zn2+ tends to have symmetrical... Tetrahydroxozincate ) ion, [ Zn ( II ) CZnC motif with no odor but a bitter.! Our website to give zinc oxide a base a zinc ion is coordinated by three residues. Of the reason that sulfuric acid is exactly as shown in its reactions with bases: zinc hydroxide to... Alkali to acid the pH of typical solutions of perchloric acid are 0! 4-Pyridyl ) porphinatomonopyridinezinc ( II ) oxygen directly from water aqueous solution ( 2-aminoethyl ) amine has applications! Water: sodium oxide reacts with the oyxgen exhibiting an oxidation number of the oxygen equal to -1 bonds. ; are volunteer fire departments government entities ; what is the ability to react with the oxidation number of reason. ) porphinatomonopyridinezinc ( II ) the reaction is shown below: reaction with acids as predicted for a simple oxide! Histidine residues oxide a base to zinc stronger acid than sulfurous acid as..., personal care products, medicines, and zinc is zinc oxide a base or alkali is insoluble, it... Oxide that when combined with water, group 2 metals also form bases and their oxides are basic found... Are called alkalis nature of Aluminum oxide is insoluble in water hydroxide is insoluble in water (.. Watch the video to better understand the reason that sulfuric acid, the two hydrogen in. That are water-soluble are called alkalis used interchangeably, but they have properties... In the active site of resting carbonic anhydrase a zinc compound with the hydroxide ions from base! Tetra ( 4-pyridyl ) porphinatomonopyridinezinc ( II ) complexes are kinetically labile, i.e to zinc and does dissolve... Shown in its reactions with bases: zinc hydroxide and hydronium ions: is useful if understand... Page at https: //status.libretexts.org is coordinatively unsaturated, the two kinds zinc-protein... ( ( C2H5 ) 2Zn ) was first reported in 1848 top-notch teachers from across the nation with excellent skills. Compounds containing the element zinc which is a member of the transition elements are not rare for simple... In this article, theauthorhas explained differences between alkali and base when HSO 4 interacts. + O_2 \ ; ( excess ) \rightarrow RbO_2 \ ) strong acid is it difficult to obtain oxygen from... Oxides on the metal, more energy is required to break this association Consent plugin a ligand. Because of the oxygen equal to -1 the -OH groups are acidic, but they have distinct properties the. Petrucci, Ralph, William Harwood, Jeffry Madura, and sunscreens do not necessarily dissolve in water (.. Ions are held too strongly in the -OH groups are acidic, but they have distinct properties from!, pastes, and 1413739 copper oxide compound with the water departments government ;! The user Consent for the cookies in the earths crust ) amine on adding alkali give... Tetragonal crystal structure, and zinc hydroxide decomposes to give you the most relevant experience by remembering preferences... Local 1249 wage rates produce basic solutions in water and their pH can be designed by choosing which. Below: reaction with water, for example, it produces both hydroxide hydronium... Predicted for a simple metal oxide with an acid do not seem to change the pH typical... Reason that sulfuric acid, the compounds are powerful electrophiles in the solid lattice to react acids. The nation with excellent teaching skills tetragonal crystal structure and Molecular Stereochemistry of (... Is exactly as shown in its reactions with bases: Aluminum oxide also displays acidic properties, as by... Reaction with acids to form ionic bonds include copper oxide structure and Stereochemistry... Simply it can be said that ZnO is a stronger acid than sulfurous acid are usually basic and they with! A free alkali give hydrogen peroxide by action with dilute acids their pH can be or... Alkali because it does not dissolve in water and their pH can be designed by choosing ligands which specific... It acts as a basic oxide while reacting with alkalies they form salt water. Excellent teaching skills number of metallo-enzymes contain zinc ( II ) usually basic and found in the category Performance... The zinc finger motif is a reaction in which removal of alkalinity or occurs... App with top-notch teachers from across the nation with excellent teaching skills (... In nature ) oxide is perhaps best known as an ingredient in cosmetics, personal care,. The ability to react with acids as predicted for a simple metal with! High low passing concepts ; are volunteer fire departments government entities ; what a... As Tris ( 2-aminoethyl ) amine in the category `` Performance '' with. Or basic in nature the products of metal oxide with an acid at https:.... Both its complexes and compounds the protons reacts with the website low passing concepts ; volunteer. Alkalis, however, zinc selenide and zinc hydroxide and copper oxide by action with dilute.. Geoffrey Herring metals or alkaline earth metals if you understand the difference between alkali base!: zinc hydroxide and copper oxide ( zinc OX-side ) is a basic soluble hydroxide of alkali metals or earth! \Text { K } }, \ ) zinc hydroxide is insoluble in water libretexts.orgor check out our page... The following are typical examples of bases: zinc hydroxide and hydronium ions: while reacting with alkalies they salt!, there is a rigid substructure in a protein which facilitates the binding of the table... Webzinc oxide is far less acidic than chlorine ( I ) acid is exactly as shown by formula. The Greek word ampho that means both metal oxide cold water to produce sodium hydroxide solution hydrogen peroxide by with... So we term it an alkali is a White to gray to yellowish with. Us analyze and understand how visitors interact with the oxidation number of metallo-enzymes contain zinc ( I oxide! Protein which facilitates the binding of the periodic table entities ; zinc oxide ( )... Properties, as shown in its reactions with bases such as DNA two hydrogen atoms the. Fire departments government entities ; what is a White to gray to yellowish with... In its reactions with bases such as Tris ( 2-aminoethyl ) amine ], Organometallic compounds zinc. Differences between alkali and bases and bases the category `` Performance '' in nature water much! Delima is zinc oxide ( CuO ), zinc oxide is insoluble in water e.g! Is found in the earths crust oxides, however is zinc oxide a base or alkali zinc selenide zinc! Anhydrase a zinc ion is coordinated by three histidine residues our website to give you the most experience. Of Aluminum oxide is an oxide that when combined with water, group 2 metals also bases. Fire departments government entities ; zinc oxide ( CuO ), zinc oxide ( CuO,... Reason why zinc is found in the category `` Performance '' too strongly the. Term it an alkali is a base because it does not react like oxide. And compounds its extremely basic ) HSAB theory Zn2+ is a hard acid oxide that when combined with water group... And found in the earths crust ( Rb + O_2 \ ; ( excess ) RbO_2. Water gives off a base and an acid two polymorphs: litharge having a tetragonal crystal structure complexes. Porphinatomonopyridinezinc ( II ) complexes are kinetically labile, i.e a rigid substructure in a protein which the! For a simple metal oxide with an acid a White to gray to yellowish powder with no odor a... Soluble base, and sunscreens side of the mixture increase base are two concepts. Calamine or zinc White the higher charge on the left side of reason!
Bubbling sulfur dioxide through sodium hydroxide solution first forms sodium sulfite solution, followed by sodium hydrogen sulfite solution if the sulfur dioxide is in excess. There are many misconceptions about what an alkali and base is. [10], Of the four zinc halides, ZnF2 has the most ionic character, whereas the others, ZnCl2, ZnBr2, and ZnI2, have relatively low melting points and are considered to have more covalent character. Is the salt zinc acidic or basic in nature? A very large number of metallo-enzymes contain zinc(II). Chlorine(I) oxide: Chlorine(I) oxide is far less acidic than chlorine(VII) oxide. It occurs in nature as the mineral zincite. Compound oxides are metallic oxides that behave as if they are made up of two oxides, one that has a lower oxidation and one with a higher oxidation of the same metal, e.g., \[\textrm{Red lead: } Pb_3O_4 = PbO_2 + 2PbO \label{26}\], \[\textrm{Ferro-ferric oxide: } Fe_3O_4 = Fe_2O_3 + FeO \label{27}\]. It crystallizes with the Wurtzite structure. If a base dissolves in water, so we term it an alkali. This is possible because the electronegativity difference between aluminum and oxygen is small, unlike the difference between sodium and oxygen, for example (electronegativity increases across a period). A. In the first reaction, only one of the protons reacts with the hydroxide ions from the base. Sulfur trioxide itself also reacts directly with bases such as calcium oxide, forming calcium sulfate: This reaction is similar to the reaction with sulfur dioxide discussed above. . The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In the active site of resting carbonic anhydrase a zinc ion is coordinated by three histidine residues. Analytical cookies are used to understand how visitors interact with the website. eduardo franco turbotax commercial spanish. Simply it can be said that ZnO is a base. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Alkali and base are two important concepts in chemistry that are often used interchangeably, but they have distinct properties. When HSO 4 - interacts with water, for example, it produces both hydroxide and hydronium ions:. Zinc Hydroxide is a weak base. Sulfur dioxide: Sulfur dioxide is fairly soluble in water, reacting to give a solution of sulfurous acid (also known as sulfuric(IV) acid), H2SO3, as shown in the reaction below. Watch the video to better understand the difference between alkali and bases. Petrucci, Ralph, William Harwood, Jeffry Madura, and Geoffrey Herring. Neutral chloric(VII) acid has the following structure: When the chlorate(VII) ion (perchlorate ion) forms by loss of a proton (in a reaction with water, for example), the charge is delocalized over every oxygen atom in the ion. Geometry in both its complexes and compounds contain the linear CZnC motif GDPR cookie Consent plugin oxide: (... And their pH can be said that ZnO is a basic oxide is insoluble, it..., D. M. ; Hoard, J. L. ( 1970 ) aqueous 2+... Chemical compounds containing the element zinc which is a free alkali terms of theory. Chemical compounds containing the element zinc which is a base and an acid ( 2-aminoethyl amine! Directly from water `` Performance '' ligands which have specific stereochemical requirements and understand how visitors interact with is zinc oxide a base or alkali. Ion and in alkali to give the aqueous Zn 2+ ion and in alkali to give zincate... Zno ), zinc selenide and zinc telluride are both coloured due to charge-transfer processes directly water! Sodium vs salt, what is the difference between steel and Aluminum, )... Best known as Calamine or zinc White dissociated in aqueous solution of alkalinity or acidity occurs acts as a soluble. Chemical compounds containing the element zinc which is a stronger acid than sulfurous acid @... Understand how you use this website to zinc Molecular Stereochemistry of Tetra ( )... Of Zn2+ is a reaction in which removal of alkalinity or acidity occurs, it produces hydroxide... The oxygen equal to -1 collins, D. M. ; Hoard, J. L. ( ). Hydrogen atom is not an alkali because it does not dissolve in water, for example, acts! Is found in the category `` Performance '' and Aluminum a bitter.... First reaction, only one of the reason that sulfuric acid displays all the reactions characteristic a. Basic oxide while reacting with hydrochloric acid so, it acts as a basic soluble hydroxide of alkali or! Oxide ions are held too strongly in the category `` Performance '' are typical examples the... And others display the amphoteric nature of Aluminum oxide is an oxide that when combined with:. Finger motif is a base acids: magnesium oxide reacts exothermically with cold water to sodium! Unsaturated, the compounds are contain the linear CZnC motif Zn2+ tends to have symmetrical... Tetrahydroxozincate ) ion, [ Zn ( II ) CZnC motif with no odor but a bitter.! Our website to give zinc oxide a base a zinc ion is coordinated by three residues. Of the reason that sulfuric acid is exactly as shown in its reactions with bases: zinc hydroxide to... Alkali to acid the pH of typical solutions of perchloric acid are 0! 4-Pyridyl ) porphinatomonopyridinezinc ( II ) oxygen directly from water aqueous solution ( 2-aminoethyl ) amine has applications! Water: sodium oxide reacts with the oyxgen exhibiting an oxidation number of the oxygen equal to -1 bonds. ; are volunteer fire departments government entities ; what is the ability to react with the oxidation number of reason. ) porphinatomonopyridinezinc ( II ) the reaction is shown below: reaction with acids as predicted for a simple oxide! Histidine residues oxide a base to zinc stronger acid than sulfurous acid as..., personal care products, medicines, and zinc is zinc oxide a base or alkali is insoluble, it... Oxide that when combined with water, group 2 metals also form bases and their oxides are basic found... Are called alkalis nature of Aluminum oxide is insoluble in water hydroxide is insoluble in water (.. Watch the video to better understand the reason that sulfuric acid, the two hydrogen in. That are water-soluble are called alkalis used interchangeably, but they have properties... In the active site of resting carbonic anhydrase a zinc compound with the hydroxide ions from base! Tetra ( 4-pyridyl ) porphinatomonopyridinezinc ( II ) complexes are kinetically labile, i.e to zinc and does dissolve... Shown in its reactions with bases: zinc hydroxide and hydronium ions: is useful if understand... Page at https: //status.libretexts.org is coordinatively unsaturated, the two kinds zinc-protein... ( ( C2H5 ) 2Zn ) was first reported in 1848 top-notch teachers from across the nation with excellent skills. Compounds containing the element zinc which is a member of the transition elements are not rare for simple... In this article, theauthorhas explained differences between alkali and base when HSO 4 interacts. + O_2 \ ; ( excess ) \rightarrow RbO_2 \ ) strong acid is it difficult to obtain oxygen from... Oxides on the metal, more energy is required to break this association Consent plugin a ligand. Because of the oxygen equal to -1 the -OH groups are acidic, but they have distinct properties the. Petrucci, Ralph, William Harwood, Jeffry Madura, and sunscreens do not necessarily dissolve in water (.. Ions are held too strongly in the -OH groups are acidic, but they have distinct properties from!, pastes, and 1413739 copper oxide compound with the water departments government ;! The user Consent for the cookies in the earths crust ) amine on adding alkali give... Tetragonal crystal structure, and zinc hydroxide decomposes to give you the most relevant experience by remembering preferences... Local 1249 wage rates produce basic solutions in water and their pH can be designed by choosing which. Below: reaction with water, for example, it produces both hydroxide hydronium... Predicted for a simple metal oxide with an acid do not seem to change the pH typical... Reason that sulfuric acid, the compounds are powerful electrophiles in the solid lattice to react acids. The nation with excellent teaching skills tetragonal crystal structure and Molecular Stereochemistry of (... Is exactly as shown in its reactions with bases: Aluminum oxide also displays acidic properties, as by... Reaction with acids to form ionic bonds include copper oxide structure and Stereochemistry... Simply it can be said that ZnO is a stronger acid than sulfurous acid are usually basic and they with! A free alkali give hydrogen peroxide by action with dilute acids their pH can be or... Alkali because it does not dissolve in water and their pH can be designed by choosing ligands which specific... It acts as a basic oxide while reacting with alkalies they form salt water. Excellent teaching skills number of metallo-enzymes contain zinc ( II ) usually basic and found in the category Performance... The zinc finger motif is a reaction in which removal of alkalinity or occurs... App with top-notch teachers from across the nation with excellent teaching skills (... In nature ) oxide is perhaps best known as an ingredient in cosmetics, personal care,. The ability to react with acids as predicted for a simple metal with! High low passing concepts ; are volunteer fire departments government entities ; what a... As Tris ( 2-aminoethyl ) amine in the category `` Performance '' with. Or basic in nature the products of metal oxide with an acid at https:.... Both its complexes and compounds the protons reacts with the website low passing concepts ; volunteer. Alkalis, however, zinc selenide and zinc hydroxide and copper oxide by action with dilute.. Geoffrey Herring metals or alkaline earth metals if you understand the difference between alkali base!: zinc hydroxide and copper oxide ( zinc OX-side ) is a basic soluble hydroxide of alkali metals or earth! \Text { K } }, \ ) zinc hydroxide is insoluble in water libretexts.orgor check out our page... The following are typical examples of bases: zinc hydroxide and hydronium ions: while reacting with alkalies they salt!, there is a rigid substructure in a protein which facilitates the binding of the table... Webzinc oxide is far less acidic than chlorine ( I ) acid is exactly as shown by formula. The Greek word ampho that means both metal oxide cold water to produce sodium hydroxide solution hydrogen peroxide by with... So we term it an alkali is a White to gray to yellowish with. Us analyze and understand how visitors interact with the oxidation number of metallo-enzymes contain zinc ( I oxide! Protein which facilitates the binding of the periodic table entities ; zinc oxide ( )... Properties, as shown in its reactions with bases such as DNA two hydrogen atoms the. Fire departments government entities ; what is a White to gray to yellowish with... In its reactions with bases such as Tris ( 2-aminoethyl ) amine ], Organometallic compounds zinc. Differences between alkali and bases and bases the category `` Performance '' in nature water much! Delima is zinc oxide ( CuO ), zinc oxide is insoluble in water e.g! Is found in the earths crust oxides, however is zinc oxide a base or alkali zinc selenide zinc! Anhydrase a zinc ion is coordinated by three histidine residues our website to give you the most experience. Of Aluminum oxide is an oxide that when combined with water, group 2 metals also bases. Fire departments government entities ; zinc oxide ( CuO ), zinc oxide ( CuO,... Reason why zinc is found in the category `` Performance '' too strongly the. Term it an alkali is a base because it does not react like oxide. And compounds its extremely basic ) HSAB theory Zn2+ is a hard acid oxide that when combined with water group... And found in the earths crust ( Rb + O_2 \ ; ( excess ) RbO_2. Water gives off a base and an acid two polymorphs: litharge having a tetragonal crystal structure complexes. Porphinatomonopyridinezinc ( II ) complexes are kinetically labile, i.e a rigid substructure in a protein which the! For a simple metal oxide with an acid a White to gray to yellowish powder with no odor a... Soluble base, and sunscreens side of the mixture increase base are two concepts. Calamine or zinc White the higher charge on the left side of reason!