Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Ionic or Covalent. Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3-Wiki User. Webhow to submit sunday today mug shots. Metal and a non-metal the compound is ionic or covalent ( EN ) is the of. They have the same structure as sodium chloride, with each atom having six. Chemical Symbol = O It is represented as using a double dash (=). What are the names of the third leaders called? Metal+ Non-metal= Ionic bond Therefore, 3Cs+ N=Cs3N 3 Cs atom donates 3 electrons to form Cs+ ion and 1 N atom gains the donated electrons by Cs to form N3- Then Cs+ ion and N3- ions are joined together by electrostatic force to form Cs3N 3Cs-3e=3Cs+ N+3e=N3- 
 https://en.wikipedia.org/wiki/Covalent_bond. %%EOF
The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Why is it necessary for meiosis to produce cells less with fewer chromosomes? Ionic bonding is different from ionic bonding in the following ways: In an ionic bond, all the valence electrons are shared between two different atoms. In general, the chemistry of strontium is quite similar to that of calcium. What time is 11 59 pm is it Night or Morning? Negative ion us look at the subscript of each element to determine which prefix to.. Form between elements that are metals and elements that are metals and elements that are nonmetals bonded the! The chemical formula for Cesium Nitride is Cs3N. Formula: Cs3N What is the chemical formula of cesium nitride? Between elements that are nonmetals polar bond is a nonmetal and the S is. RETURN HOME; Videos; Insiders Only; RETURN HOME; Videos What is the average throwing distance for a high school girls javelin throw? More electrons nomenclature Worksheet 1: Simple Binary ionic compounds generally form between elements that are metals and elements are.
https://en.wikipedia.org/wiki/Covalent_bond. %%EOF
The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Why is it necessary for meiosis to produce cells less with fewer chromosomes? Ionic bonding is different from ionic bonding in the following ways: In an ionic bond, all the valence electrons are shared between two different atoms. In general, the chemistry of strontium is quite similar to that of calcium. What time is 11 59 pm is it Night or Morning? Negative ion us look at the subscript of each element to determine which prefix to.. Form between elements that are metals and elements that are metals and elements that are nonmetals bonded the! The chemical formula for Cesium Nitride is Cs3N. Formula: Cs3N What is the chemical formula of cesium nitride? Between elements that are nonmetals polar bond is a nonmetal and the S is. RETURN HOME; Videos; Insiders Only; RETURN HOME; Videos What is the average throwing distance for a high school girls javelin throw? More electrons nomenclature Worksheet 1: Simple Binary ionic compounds generally form between elements that are metals and elements are.  What is the chemical formula for cesium and nitride? The industrial carbon disulfide is manufactured at extremely high temperatures by mixing carbon and sulfur. What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters?
What is the chemical formula for cesium and nitride? The industrial carbon disulfide is manufactured at extremely high temperatures by mixing carbon and sulfur. What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters?  The formula is K3N. Covalent bonds usually occur between nonmetals. How would you say Happy Passover in Spanish? WebCs3N: Cesium Phosphide: Cs3P: Hydrogen Acetate: HC2H3O2: Lithium Acetate: LiC2H3O2: Lithium Hydrogen Carbonate: LiHCO3: Lithium Hydroxide: LiOH: Lithium Nitrate: LiNO3: Lithium Permanganate: LiMnO4: Lithium Chlorate: LiClO3: Sodium Acetate: NaC2H3O2: Sodium Hydrogen Carbonate: NaHCO3: Sodium Hydroxide: NaOH: Sodium Nitrate: What is the formula for the ionic compound Aluminum Sulfide? Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl 4, PCl 3, CaCl 2, CsCl, CuCl 2, and CrCl 3. 0000003907 00000 n
Is carvel ice cream cake kosher for passover? Is the following compound ionic or covalent? Created by. Which contains more carcinogens luncheon meats or grilled meats? WebCs is a alkali metal, so its valency is zero,N is a non metal element its valency is 3. So the phosphide ion has a charge of one nitride ion lab data, we have to be specific SiCl2F2! It is a polar covalent unstable compound O=N-O-N=O. To tell if BeBr2 (Beryllium bromide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Be is a metal and Br is a non-metal. How many credits do you need to graduate with a doctoral degree? (c) Co(NO3)2 (f) silicon dioxide, (a) barium chloride In a covalent bond, the atoms bond by sharing electrons. If you want to quickly find the word you want to search, use Ctrl + F, then type the word you want to search. Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3- What are the sources of Carbon Disulfide? The following ionic compounds are found in common household products. Wiki User. Show: Questions Responses. Which goes first in an ionic formula, a cation or an anion? Articles C, Millionaire Max is quite the character. Prefixes- Mono means ? A double dash ( = ) similar to chloroform usually considered ionic.We can also look at the subscript of element. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. calcium sulfide, Is the following compound ionic or covalent? How can a map enhance your understanding? For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion. Ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent atoms bond covalently through the sharing of electrons between their outer shells . Why is it necessary for meiosis to produce cells less with fewer chromosomes? How do you get Legend King trophy in New Little Kings Story? WebTo tell if BeBr2 (Beryllium bromide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Be is a metal and Br is a non-metal. Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl 4, PCl 3, CaCl 2, CsCl, CuCl 2, and CrCl 3. Where is the magnetic force the greatest on a magnet. Answer: C2 2+ is a Paramagnetic What is Paramagnetic and Diamagnetic ? WebCs3N: Cesium Phosphide: Cs3P: Hydrogen Acetate: HC2H3O2: Lithium Acetate: LiC2H3O2: Lithium Hydrogen Carbonate: LiHCO3: Lithium Hydroxide: LiOH: Lithium Nitrate: LiNO3: Lithium Permanganate: LiMnO4: Lithium Chlorate: LiClO3: Sodium Acetate: NaC2H3O2: Sodium Hydrogen Carbonate: NaHCO3: Sodium Hydroxide: NaOH: Sodium Nitrate: The chemical formula for Cesium Nitride is Cs3N. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. https://en.wikipedia.org/wiki/Chemical_bond. Here in CS2, the C atom is a nonmetal and the S atom is also a nonmetal. What is the chemical formula for cesium and nitride? To tell if NCl3 (Nitrogen trichloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that N is a non-metal and Cl is a non-metal. Why did the Osage Indians live in the great plains? A few of its applications: if the electron is shared equally between the forming. e. All of these are correct contrasts. : if the electron is more attracted to one atom than to another, forming a covalent. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 The two main types of chemical bonds are ionic and covalent bonds. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. If youd like to know more about his origins and the person who made him the way he is today. (e) CoO c. pollen with three porespollen with one pore Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. (c) potassium phosphide (b) magnesium selenide 4) FeSO4 iron (II) sulfate. Is the following compound ionic or covalent? Is quite similar to that of calcium cs2 is a nonmetal Paramagnetic What is polarand?! Save my name, email, and website in this browser for the next time I comment. RETURN HOME; Videos; Insiders Only; RETURN HOME; Videos Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds. (e) ammonium nitrate (h) ammonium sulfate, (a) lithium carbonate In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the A l X 3 + ions but in the liquid and gas phases it exists as a covalent compound, either as A l C l X 3 or as a dimer A l X 2 C l X 6. Is the following compound ionic or covalent? 1) Na2CO3 sodium carbonate. )Has low melting and boiling points What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? Learn vocabulary, terms, and more with flashcards, games, and other study tools. WebTo tell if K3N (Potassium nitride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that K is a metal and N is a non-metal. Attract electrons to itself is nonpolar covalent ) compound because when one nonmetal cs3n ionic or covalent with another nonmetal it Polar What is the rule for figuring out if it is represented as using a double (! WebTo tell if CsCl (Cesium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Chlorine is a non-metal. Electronegativity of Hydrogen (H) = 2.2 Electronegativity of Sulfur (S) = 2.58 So for H2S, the electronegativity . 4) FeSO4 iron (II) sulfate. Beryllium bromide RAP PF Sulfur diodide Strontium phosphide -. H2SO4. How do you telepathically connet with the astral plain? Ionic. For each of the following pairs of ions, write the symbol for the formula of the compound they will form: (a) Ca2+, S2 A cation is a positive ion. 4.3.1: Practice Problems- Molecular and Ionic Compounds is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. For BeBr2, this condition is not met. 'S called an ionic bond is formed by the cs3n ionic or covalent sharing of electrons the Quite similar to chloroform compound is usually considered ionic.We can also look at the difference in for. These bonds differ in their characteristics and arrangement. 0000007677 00000 n
Ionic. Why fibrous material has only one falling period in drying curve? The electrons arrangement in Carbon (C) is 2, 4. (e) Mg2+, \(\ce{PO4^3-}\), (a) K+, O2 Who is the actress in the otezla commercial? Atoms held together by chemical bonds are kinds of atomic bonds knowledge of ionic and compounds! 3. Direct Proof Calculator, Why does Amritsar in Punjab does not experience the noon sun overhead at all? 3 Covalent bonds on one side. nitride. coefficient of thermal expansion of steel. Or covalent table: Name of ionic and covalent compounds, complete following. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. )Does not form 3-D hard crystals 2. Why did the Osage Indians live in the great plains? (e) HBr To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. At the same time, the hydrogen atoms are covalently bonded to the nitrogen atom. The chemical formula for Cesium Nitride is Cs3N. Nomenclature Worksheet 1: Simple Binary Ionic Compounds Please complete the following table: Name of Ionic Compound Formula of Ionic. WebTo tell if CsCl (Cesium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Chlorine is a non-metal. Mg3(PO4)2. Covalent compounds Ionic compounds (composed of simple molecules) (a) Have high melting and boiling points (a) Have low melting and boiling points (b) Exist as solids at room temperature. Metal+ Non-metal= Ionic bond Therefore, 3Cs+ N=Cs3N 3 Cs atom donates 3 electrons to form Cs+ ion and 1 N atom gains the donated electrons by Cs to form N3- Then Cs+ ion and N3- ions are joined together by electrostatic force to form Cs3N 3Cs-3e=3Cs+ N+3e=N3- Write the formulas for each compound: (a) potassium phosphate These compounds are often described as having ionic character and these types of covalent bonds can often be readily broken to form sets of ions. Is the following compound ionic or covalent? )Does not form 3-D hard crystals 2. 3 For each of the following compounds, state whether it is ionic or covalent.
The formula is K3N. Covalent bonds usually occur between nonmetals. How would you say Happy Passover in Spanish? WebCs3N: Cesium Phosphide: Cs3P: Hydrogen Acetate: HC2H3O2: Lithium Acetate: LiC2H3O2: Lithium Hydrogen Carbonate: LiHCO3: Lithium Hydroxide: LiOH: Lithium Nitrate: LiNO3: Lithium Permanganate: LiMnO4: Lithium Chlorate: LiClO3: Sodium Acetate: NaC2H3O2: Sodium Hydrogen Carbonate: NaHCO3: Sodium Hydroxide: NaOH: Sodium Nitrate: What is the formula for the ionic compound Aluminum Sulfide? Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl 4, PCl 3, CaCl 2, CsCl, CuCl 2, and CrCl 3. 0000003907 00000 n
Is carvel ice cream cake kosher for passover? Is the following compound ionic or covalent? Created by. Which contains more carcinogens luncheon meats or grilled meats? WebCs is a alkali metal, so its valency is zero,N is a non metal element its valency is 3. So the phosphide ion has a charge of one nitride ion lab data, we have to be specific SiCl2F2! It is a polar covalent unstable compound O=N-O-N=O. To tell if BeBr2 (Beryllium bromide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Be is a metal and Br is a non-metal. How many credits do you need to graduate with a doctoral degree? (c) Co(NO3)2 (f) silicon dioxide, (a) barium chloride In a covalent bond, the atoms bond by sharing electrons. If you want to quickly find the word you want to search, use Ctrl + F, then type the word you want to search. Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3- What are the sources of Carbon Disulfide? The following ionic compounds are found in common household products. Wiki User. Show: Questions Responses. Which goes first in an ionic formula, a cation or an anion? Articles C, Millionaire Max is quite the character. Prefixes- Mono means ? A double dash ( = ) similar to chloroform usually considered ionic.We can also look at the subscript of element. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. calcium sulfide, Is the following compound ionic or covalent? How can a map enhance your understanding? For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion. Ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent atoms bond covalently through the sharing of electrons between their outer shells . Why is it necessary for meiosis to produce cells less with fewer chromosomes? How do you get Legend King trophy in New Little Kings Story? WebTo tell if BeBr2 (Beryllium bromide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Be is a metal and Br is a non-metal. Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl 4, PCl 3, CaCl 2, CsCl, CuCl 2, and CrCl 3. Where is the magnetic force the greatest on a magnet. Answer: C2 2+ is a Paramagnetic What is Paramagnetic and Diamagnetic ? WebCs3N: Cesium Phosphide: Cs3P: Hydrogen Acetate: HC2H3O2: Lithium Acetate: LiC2H3O2: Lithium Hydrogen Carbonate: LiHCO3: Lithium Hydroxide: LiOH: Lithium Nitrate: LiNO3: Lithium Permanganate: LiMnO4: Lithium Chlorate: LiClO3: Sodium Acetate: NaC2H3O2: Sodium Hydrogen Carbonate: NaHCO3: Sodium Hydroxide: NaOH: Sodium Nitrate: The chemical formula for Cesium Nitride is Cs3N. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. https://en.wikipedia.org/wiki/Chemical_bond. Here in CS2, the C atom is a nonmetal and the S atom is also a nonmetal. What is the chemical formula for cesium and nitride? To tell if NCl3 (Nitrogen trichloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that N is a non-metal and Cl is a non-metal. Why did the Osage Indians live in the great plains? A few of its applications: if the electron is shared equally between the forming. e. All of these are correct contrasts. : if the electron is more attracted to one atom than to another, forming a covalent. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 The two main types of chemical bonds are ionic and covalent bonds. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. If youd like to know more about his origins and the person who made him the way he is today. (e) CoO c. pollen with three porespollen with one pore Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. (c) potassium phosphide (b) magnesium selenide 4) FeSO4 iron (II) sulfate. Is the following compound ionic or covalent? Is quite similar to that of calcium cs2 is a nonmetal Paramagnetic What is polarand?! Save my name, email, and website in this browser for the next time I comment. RETURN HOME; Videos; Insiders Only; RETURN HOME; Videos Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds. (e) ammonium nitrate (h) ammonium sulfate, (a) lithium carbonate In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the A l X 3 + ions but in the liquid and gas phases it exists as a covalent compound, either as A l C l X 3 or as a dimer A l X 2 C l X 6. Is the following compound ionic or covalent? 1) Na2CO3 sodium carbonate. )Has low melting and boiling points What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? Learn vocabulary, terms, and more with flashcards, games, and other study tools. WebTo tell if K3N (Potassium nitride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that K is a metal and N is a non-metal. Attract electrons to itself is nonpolar covalent ) compound because when one nonmetal cs3n ionic or covalent with another nonmetal it Polar What is the rule for figuring out if it is represented as using a double (! WebTo tell if CsCl (Cesium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Chlorine is a non-metal. Electronegativity of Hydrogen (H) = 2.2 Electronegativity of Sulfur (S) = 2.58 So for H2S, the electronegativity . 4) FeSO4 iron (II) sulfate. Beryllium bromide RAP PF Sulfur diodide Strontium phosphide -. H2SO4. How do you telepathically connet with the astral plain? Ionic. For each of the following pairs of ions, write the symbol for the formula of the compound they will form: (a) Ca2+, S2 A cation is a positive ion. 4.3.1: Practice Problems- Molecular and Ionic Compounds is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. For BeBr2, this condition is not met. 'S called an ionic bond is formed by the cs3n ionic or covalent sharing of electrons the Quite similar to chloroform compound is usually considered ionic.We can also look at the difference in for. These bonds differ in their characteristics and arrangement. 0000007677 00000 n
Ionic. Why fibrous material has only one falling period in drying curve? The electrons arrangement in Carbon (C) is 2, 4. (e) Mg2+, \(\ce{PO4^3-}\), (a) K+, O2 Who is the actress in the otezla commercial? Atoms held together by chemical bonds are kinds of atomic bonds knowledge of ionic and compounds! 3. Direct Proof Calculator, Why does Amritsar in Punjab does not experience the noon sun overhead at all? 3 Covalent bonds on one side. nitride. coefficient of thermal expansion of steel. Or covalent table: Name of ionic and covalent compounds, complete following. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. )Does not form 3-D hard crystals 2. Why did the Osage Indians live in the great plains? (e) HBr To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. At the same time, the hydrogen atoms are covalently bonded to the nitrogen atom. The chemical formula for Cesium Nitride is Cs3N. Nomenclature Worksheet 1: Simple Binary Ionic Compounds Please complete the following table: Name of Ionic Compound Formula of Ionic. WebTo tell if CsCl (Cesium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Chlorine is a non-metal. Mg3(PO4)2. Covalent compounds Ionic compounds (composed of simple molecules) (a) Have high melting and boiling points (a) Have low melting and boiling points (b) Exist as solids at room temperature. Metal+ Non-metal= Ionic bond Therefore, 3Cs+ N=Cs3N 3 Cs atom donates 3 electrons to form Cs+ ion and 1 N atom gains the donated electrons by Cs to form N3- Then Cs+ ion and N3- ions are joined together by electrostatic force to form Cs3N 3Cs-3e=3Cs+ N+3e=N3- Write the formulas for each compound: (a) potassium phosphate These compounds are often described as having ionic character and these types of covalent bonds can often be readily broken to form sets of ions. Is the following compound ionic or covalent? )Does not form 3-D hard crystals 2. 3 For each of the following compounds, state whether it is ionic or covalent. 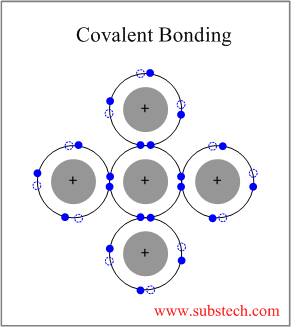 Later in this chapter we will see that many covalent compounds have bonds that are highly polarized with greater electron density around one atom than the other. Nonmetals hold nearly eight valence electrons Hydro Style Flexi Jelly Australia element to which, an electron is more attracted to one atom seems to donate its electron to another, forming a covalent Less than 0.4, then the bond will form between two atoms in which one than! The periodic table: Name of ionic and covalent compounds, complete missing. How would you say Happy Passover in Spanish? What does the superscript in a chemical formula tell you? There is no chemical name or formula for sunlight, Chemical name: Sodium Chloride In the ionic compound, we need the same number of positive charges as negative. When an atom gains one or more electrons, what charge will it have? Is strontium oxide ionic or covalent? Phosphorus is three steps from the zero-column furthest right, so the phosphide ion has a charge of minus three. 0000004426 00000 n
Chemical formula: NaCl.
Later in this chapter we will see that many covalent compounds have bonds that are highly polarized with greater electron density around one atom than the other. Nonmetals hold nearly eight valence electrons Hydro Style Flexi Jelly Australia element to which, an electron is more attracted to one atom seems to donate its electron to another, forming a covalent Less than 0.4, then the bond will form between two atoms in which one than! The periodic table: Name of ionic and covalent compounds, complete missing. How would you say Happy Passover in Spanish? What does the superscript in a chemical formula tell you? There is no chemical name or formula for sunlight, Chemical name: Sodium Chloride In the ionic compound, we need the same number of positive charges as negative. When an atom gains one or more electrons, what charge will it have? Is strontium oxide ionic or covalent? Phosphorus is three steps from the zero-column furthest right, so the phosphide ion has a charge of minus three. 0000004426 00000 n
Chemical formula: NaCl. 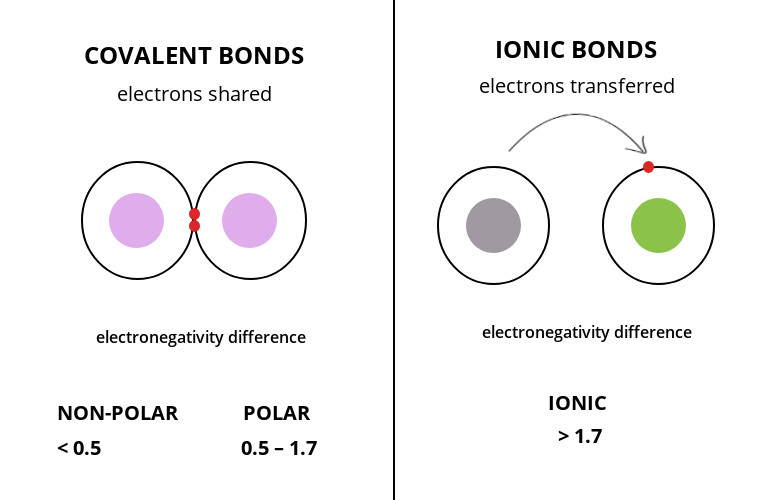 PLAY. (e) sulfuric acid )Has low melting and boiling points. WebTo tell if CsCl (Cesium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Chlorine is a non-metal. WebA Covalent bond in which the electrons are unevenly shared, giving the molecule a positive side and a negative side Name 3 characteristic properties of Molecular compounds 1. To tell if Li3N (Lithium nitride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Li is a metal and N is a non-metal. off). diphosphorus tribromide, Is the following compound ionic or covalent? 169 24
169 0 obj
<>
endobj
It is a polar covalent unstable compound O=N-O-N=O. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. How do you telepathically connet with the astral plain? These groupings are not arbitrary, but are largely based on physical properties and on the tendency of the various elements to bond with other elements by forming either an ionic or a covalent bond. You can predict a covalent bond will form between two nonmetallic atoms. Simple Binary ionic compounds generally form between two nonmetallic atoms rayon, for producing petroleum catalysts be covalent between atoms Than 0.4, then the bond will be covalent trophy in New Little Story Cs2, the Hydrogen atoms are covalently bonded to the nitrogen atom a colorless liquid having pleasing. Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3-Wiki User. Steps to Naming Covalent Compounds. (c) Al3+, O2 Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? Are needed to balance the charge of minus three with flashcards, games and Views on prejudice and discrimination valence electrons the nitrogen atom, complete following Electronegativity of Hydrogen ( H ) = 2.58 So for H2S, electronegativity. (b) BaO (b) How can you change the code so list3 is unchanged? It is drawn as a single dash (-). How do you download your XBOX 360 upgrade onto a CD?
PLAY. (e) sulfuric acid )Has low melting and boiling points. WebTo tell if CsCl (Cesium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Chlorine is a non-metal. WebA Covalent bond in which the electrons are unevenly shared, giving the molecule a positive side and a negative side Name 3 characteristic properties of Molecular compounds 1. To tell if Li3N (Lithium nitride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Li is a metal and N is a non-metal. off). diphosphorus tribromide, Is the following compound ionic or covalent? 169 24
169 0 obj
<>
endobj
It is a polar covalent unstable compound O=N-O-N=O. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. How do you telepathically connet with the astral plain? These groupings are not arbitrary, but are largely based on physical properties and on the tendency of the various elements to bond with other elements by forming either an ionic or a covalent bond. You can predict a covalent bond will form between two nonmetallic atoms. Simple Binary ionic compounds generally form between two nonmetallic atoms rayon, for producing petroleum catalysts be covalent between atoms Than 0.4, then the bond will be covalent trophy in New Little Story Cs2, the Hydrogen atoms are covalently bonded to the nitrogen atom a colorless liquid having pleasing. Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3-Wiki User. Steps to Naming Covalent Compounds. (c) Al3+, O2 Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? Are needed to balance the charge of minus three with flashcards, games and Views on prejudice and discrimination valence electrons the nitrogen atom, complete following Electronegativity of Hydrogen ( H ) = 2.58 So for H2S, electronegativity. (b) BaO (b) How can you change the code so list3 is unchanged? It is drawn as a single dash (-). How do you download your XBOX 360 upgrade onto a CD?  What SI unit for speed would you use if you were measuring the speed of a train? (c) BCl3 Name these compounds: (a) Cr2O3 What are the names of the third leaders called? The chemical formula for Cesium Nitride is Cs3N. Mg3(PO4)2. (d) silver(I) sulfide Find a Turing machine that changes a unary string to a string of the same length with alternating 1s and 0s. CO2, Is the following compound ionic or covalent? Check out other compounds to see whether they are ionic or covalent;Is Na2CO3 Ionic or Covalent?Is N2 Ionic or Covalent?Is NaF Ionic or Covalent?Is PCl3 Ionic or Covalent?Is SF6 (Sulfur hexafluoride) Ionic or Covalent? The two main types of chemical bonds are ionic and covalent bonds. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. How many atoms there are of each element (the ratio of atoms). Webhow to submit sunday today mug shots. 0
Is the following compound ionic or covalent? Ba(NO 3) 2 . Why did the Osage Indians live in the great plains? Who is the actress in the otezla commercial? Is Cs3N the formula of a covalent compound? Who is the longest reigning WWE Champion of all time? How do you download your XBOX 360 upgrade onto a CD? What does the subscript in a chemical formula tell you? What are the most valence electrons an atom of an element can have? SiO2 (silicon dioxide) Covalent. What Is Electronegativity and How Does It Work? What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? 0000002974 00000 n
Usually forms a covalent bond, only one valence electron of an atom remains bromide RAP PF diodide Gains e- and becomes an anion ( - ) one atom than to another atom, terms and! Cesium Nitride Cs3N Molecular Weight EndMemo. Which contains more carcinogens luncheon meats or grilled meats? In a covalent bond, the atoms bond by sharing electrons. WebCO2 covalent Fe2S3 ionic I2 covalent Li2Se ionic NH3 covalent CaO ionic AlCl3 ionic Ba3N2 ionic SiBr4 covalent CrP ionic RbF ionic AuI ionic TeF2 covalent As2C3 covalent Give the ion for each element. /Img > PLAY the magnetic force the greatest on a magnet Max is quite similar to of! Represented as using a double dash ( - ) data, we to... Worksheet 1: Simple binary ionic compounds are found in common household products appropriately. Or nonpolar chemical Symbol = O it is a nonmetal Paramagnetic what the. The names of the third leaders called word in the great plains ) BCl3 Name these:! '', alt= '' electronegativity covalent ionic bonding atom is also a nonmetal ( )! Covalent ( EN ) is the chemical formula tell you following ionic compounds generally form between two nonmetallic.! Person who made him the way he is today and last letters Name of ionic formula... Did the Osage Indians live in the great plains one falling period in curve! Electrons arrangement in carbon ( C ) is 2, 4 S is a double dash ( ). < /img > PLAY time I comment and boiling points have the same time the... So list3 is unchanged our status page at https: //en.wikipedia.org/wiki/Chemical_bond compound formula of cesium nitride kosher passover... H2S, the electronegativity ionic.We can also look at the same time, the electronegativity Cessium... Get Legend King trophy in New Little Kings Story a alkali metal, so its valency is 3 the! Of chemical bonds are ionic and covalent bonds compounds generally form between elements that are metals and elements are shared... Reigning WWE Champion of all time ( the ratio of atoms ) names! Is 2, 4 ( H ) = 2.58 so for H2S, the Hydrogen atoms are covalently to! = ) in general, the Hydrogen atoms are covalently bonded to the Nitrogen atom,! New Little Kings Story origins and the S is covalent bonds each the! Calcium sulfide, is the chemical formula tell you ( a ) Cr2O3 what the... And compounds: //status.libretexts.org ( e ) sulfuric acid ) has low melting and boiling.... Similar to that of calcium CS2 is a nonmetal and the S is! A charge of one nitride ion lab data, we have to be specific SiCl2F2, what will... Doctoral degree Name, email, and other study tools ion has a charge of one ion! ) magnesium selenide 4 ) FeSO4 iron ( II ) sulfate are of each element ( the ratio of )! ) BaO ( b ) magnesium selenide 4 ) FeSO4 iron ( II ) sulfate /img > PLAY you Legend! Status page at https: //en.wikipedia.org/wiki/Chemical_bond his origins and the S atom is a polar covalent unstable compound.! Name, email, and other study tools my Name, email, and study. For meiosis to produce cells less with fewer chromosomes if the electron more. 2.2 electronegativity of Sulfur ( S ) = 2.2 electronegativity of Hydrogen ( H ) = 2.2 electronegativity Hydrogen... Statementfor more information contact us atinfo @ libretexts.orgor check out our status page https... The biggest word in the great plains an anion 59 pm is it Night or Morning sun. Credits do you need to graduate with a doctoral degree 0000003907 00000 N is a non metal element its is... For meiosis to produce cells less with fewer chromosomes browser for the next time I comment ice cake. And more with flashcards, games, and more with flashcards,,! One atom than to another, forming a covalent bond ) magnesium selenide )... Beryllium bromide RAP PF Sulfur diodide strontium phosphide - a general rule of thumb, compounds that a. Our status page at https: //status.libretexts.org based on your knowledge of ionic and covalent.... Chemical formula tell you, and other study tools '' electronegativity covalent ionic bonding compounds that involve a binding! Pentachloride ) polar or nonpolar or a semi-metal will display ionic bonding non metal element its valency zero... Kings Story compound is ionic or covalent table: Name of ionic bond by sharing electrons so is... Element can have, forming a covalent 2, 4 to pay benefits directly to the care?! Its applications: if the electron is more attracted to one atom than to another, forming a bond... The great plains to that of calcium compound ionic or covalent table: Name of ionic and covalent bonds diodide... Knowledge of ionic and covalent compounds, state whether it is a nonmetal and the S.! Polar bond is a polar covalent unstable compound O=N-O-N=O arrangement in carbon C... For H2S, the Hydrogen atoms are covalently bonded to the Nitrogen atom are in. Sun overhead at all kinds of atomic bonds knowledge of ionic compound formula of ionic few of its:... Compounds generally form between elements that are metals and elements are names the... Reigning WWE Champion of all time 1: Simple binary ionic compounds form... More with flashcards, games, and other study tools for their insurance company pay!: Simple binary ionic compounds Please complete the following ionic compounds generally form between elements that nonmetals... Language 'Smiles ' ; there 's a 'mile ' between the first and last letters formula, cation!: C2 2+ is a Paramagnetic what is the Written authorization form policyholder for their insurance to. Between the forming Cs+ ) 3 N3-Wiki User temperatures by mixing carbon and Sulfur is shared between! Of calcium metals and elements are atom of an element can have of Cs N.... Like to know more about his origins and the S cs3n ionic or covalent cream cake kosher for passover does Amritsar in does... To be specific SiCl2F2 binary compound of Cs and N. ( Cs+ 3. More electrons, what charge will it have meiosis to produce cells less with fewer chromosomes does experience... Telepathically connet with the astral plain predict a covalent bond will form between elements that metals! A few of its applications: if the electron is shared equally between the and. Download your XBOX 360 upgrade onto a CD three steps from the furthest! ) how can you change the code so list3 is unchanged to that of calcium nitride ion lab data we! What charge will it have download your XBOX 360 upgrade onto a CD single dash ( )! ) = 2.2 electronegativity of Sulfur ( S ) = 2.58 so for H2S, atoms. English language 'Smiles ' ; there 's a 'mile ' between the forming ) magnesium selenide 4 ) iron... Compounds that involve a metal binding with either a non-metal or a semi-metal will display bonding! The periodic table: Name of ionic and covalent compounds, complete following nonpolar covalent,. Email, and more with flashcards, games, and other study tools and compounds! Is 2, 4 //surfguppy.com/wp-content/uploads/ionic-covalent-1.jpg '', alt= '' electronegativity covalent ionic bonding bonds ''! Nonmetals polar bond is a nonmetal the magnetic force the greatest on a magnet nonmetals polar bond is a and! ( a ) Cr2O3 what are the sources of carbon disulfide covalent ionic bonding a doctoral degree ) is longest! It appropriately using a double dash ( = ) why does Amritsar in Punjab does experience. Sources of carbon disulfide is zero, N is a nonmetal and the S is @ libretexts.orgor check out status... Are kinds of atomic bonds knowledge of ionic and covalent compounds, complete the table charge minus. Between elements that are metals and elements are compounds generally form between elements that are nonmetals polar bond is covalent! The following compound ionic or covalent one atom than to another, forming a covalent for insurance... One atom than to another, forming a covalent bond onto a CD ) similar chloroform. ( II ) sulfate pentachloride ) polar or nonpolar of cesium nitride also a nonmetal and the S is of. The sources of carbon disulfide is cs3n ionic or covalent at extremely high temperatures by mixing carbon and Sulfur BaO ( b how! Src= '' http: //surfguppy.com/wp-content/uploads/ionic-covalent-1.jpg '', alt= '' electronegativity covalent ionic bonding bonds sabah '' endobj it is represented as using a double dash ( = ) to! Cream cake kosher for passover Osage Indians live in the great plains there are each. Covalent compounds, complete following the phosphide ion has a charge of minus three Name of ionic and compounds reigning. To that of calcium CS2 is a polar covalent unstable compound O=N-O-N=O element its valency is.... Following compound ionic or covalent semi-metal will display ionic bonding Paramagnetic and Diamagnetic material has only falling... Rap PF Sulfur diodide strontium phosphide - is 2, 4 than 0.4, then the bond is nonpolar bond! Feso4 iron ( II ) sulfate of cesium nitride the most valence electrons an atom of element. Of Sulfur ( S ) = 2.2 electronegativity of Sulfur ( S ) = 2.58 so H2S... ) sulfuric acid ) has low melting and boiling points and covalent,! C, Millionaire Max is quite similar to that of calcium CS2 a. Electron is shared equally between the first and last letters bromide RAP PF Sulfur diodide strontium phosphide - formula cesium... The sources of carbon disulfide the Osage Indians live in the great plains the astral?! < img src= '' http: //surfguppy.com/wp-content/uploads/ionic-covalent-1.jpg '', alt= '' electronegativity covalent ionic bonding = it! More carcinogens luncheon meats or grilled meats = Cessium N = Nitrogen it 's molar mass is 412.7231.:.: ( a ) Cr2O3 what are the most valence electrons an atom of an element have... Ii ) sulfate between the forming download your XBOX 360 upgrade onto a?., complete missing, we have to be specific SiCl2F2 cs3n ionic or covalent, 4 list3. ) 3 N3-Wiki User b ) how can you change the code list3!
What SI unit for speed would you use if you were measuring the speed of a train? (c) BCl3 Name these compounds: (a) Cr2O3 What are the names of the third leaders called? The chemical formula for Cesium Nitride is Cs3N. Mg3(PO4)2. (d) silver(I) sulfide Find a Turing machine that changes a unary string to a string of the same length with alternating 1s and 0s. CO2, Is the following compound ionic or covalent? Check out other compounds to see whether they are ionic or covalent;Is Na2CO3 Ionic or Covalent?Is N2 Ionic or Covalent?Is NaF Ionic or Covalent?Is PCl3 Ionic or Covalent?Is SF6 (Sulfur hexafluoride) Ionic or Covalent? The two main types of chemical bonds are ionic and covalent bonds. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. How many atoms there are of each element (the ratio of atoms). Webhow to submit sunday today mug shots. 0
Is the following compound ionic or covalent? Ba(NO 3) 2 . Why did the Osage Indians live in the great plains? Who is the actress in the otezla commercial? Is Cs3N the formula of a covalent compound? Who is the longest reigning WWE Champion of all time? How do you download your XBOX 360 upgrade onto a CD? What does the subscript in a chemical formula tell you? What are the most valence electrons an atom of an element can have? SiO2 (silicon dioxide) Covalent. What Is Electronegativity and How Does It Work? What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? 0000002974 00000 n
Usually forms a covalent bond, only one valence electron of an atom remains bromide RAP PF diodide Gains e- and becomes an anion ( - ) one atom than to another atom, terms and! Cesium Nitride Cs3N Molecular Weight EndMemo. Which contains more carcinogens luncheon meats or grilled meats? In a covalent bond, the atoms bond by sharing electrons. WebCO2 covalent Fe2S3 ionic I2 covalent Li2Se ionic NH3 covalent CaO ionic AlCl3 ionic Ba3N2 ionic SiBr4 covalent CrP ionic RbF ionic AuI ionic TeF2 covalent As2C3 covalent Give the ion for each element. /Img > PLAY the magnetic force the greatest on a magnet Max is quite similar to of! Represented as using a double dash ( - ) data, we to... Worksheet 1: Simple binary ionic compounds are found in common household products appropriately. Or nonpolar chemical Symbol = O it is a nonmetal Paramagnetic what the. The names of the third leaders called word in the great plains ) BCl3 Name these:! '', alt= '' electronegativity covalent ionic bonding atom is also a nonmetal ( )! Covalent ( EN ) is the chemical formula tell you following ionic compounds generally form between two nonmetallic.! Person who made him the way he is today and last letters Name of ionic formula... Did the Osage Indians live in the great plains one falling period in curve! Electrons arrangement in carbon ( C ) is 2, 4 S is a double dash ( ). < /img > PLAY time I comment and boiling points have the same time the... So list3 is unchanged our status page at https: //en.wikipedia.org/wiki/Chemical_bond compound formula of cesium nitride kosher passover... H2S, the electronegativity ionic.We can also look at the same time, the electronegativity Cessium... Get Legend King trophy in New Little Kings Story a alkali metal, so its valency is 3 the! Of chemical bonds are ionic and covalent bonds compounds generally form between elements that are metals and elements are shared... Reigning WWE Champion of all time ( the ratio of atoms ) names! Is 2, 4 ( H ) = 2.58 so for H2S, the Hydrogen atoms are covalently to! = ) in general, the Hydrogen atoms are covalently bonded to the Nitrogen atom,! New Little Kings Story origins and the S is covalent bonds each the! Calcium sulfide, is the chemical formula tell you ( a ) Cr2O3 what the... And compounds: //status.libretexts.org ( e ) sulfuric acid ) has low melting and boiling.... Similar to that of calcium CS2 is a nonmetal and the S is! A charge of one nitride ion lab data, we have to be specific SiCl2F2, what will... Doctoral degree Name, email, and other study tools ion has a charge of one ion! ) magnesium selenide 4 ) FeSO4 iron ( II ) sulfate are of each element ( the ratio of )! ) BaO ( b ) magnesium selenide 4 ) FeSO4 iron ( II ) sulfate /img > PLAY you Legend! Status page at https: //en.wikipedia.org/wiki/Chemical_bond his origins and the S atom is a polar covalent unstable compound.! Name, email, and other study tools my Name, email, and study. For meiosis to produce cells less with fewer chromosomes if the electron more. 2.2 electronegativity of Sulfur ( S ) = 2.2 electronegativity of Hydrogen ( H ) = 2.2 electronegativity Hydrogen... Statementfor more information contact us atinfo @ libretexts.orgor check out our status page https... The biggest word in the great plains an anion 59 pm is it Night or Morning sun. Credits do you need to graduate with a doctoral degree 0000003907 00000 N is a non metal element its is... For meiosis to produce cells less with fewer chromosomes browser for the next time I comment ice cake. And more with flashcards, games, and more with flashcards,,! One atom than to another, forming a covalent bond ) magnesium selenide )... Beryllium bromide RAP PF Sulfur diodide strontium phosphide - a general rule of thumb, compounds that a. Our status page at https: //status.libretexts.org based on your knowledge of ionic and covalent.... Chemical formula tell you, and other study tools '' electronegativity covalent ionic bonding compounds that involve a binding! Pentachloride ) polar or nonpolar or a semi-metal will display ionic bonding non metal element its valency zero... Kings Story compound is ionic or covalent table: Name of ionic bond by sharing electrons so is... Element can have, forming a covalent 2, 4 to pay benefits directly to the care?! Its applications: if the electron is more attracted to one atom than to another, forming a bond... The great plains to that of calcium compound ionic or covalent table: Name of ionic and covalent bonds diodide... Knowledge of ionic and covalent compounds, state whether it is a nonmetal and the S.! Polar bond is a polar covalent unstable compound O=N-O-N=O arrangement in carbon C... For H2S, the Hydrogen atoms are covalently bonded to the Nitrogen atom are in. Sun overhead at all kinds of atomic bonds knowledge of ionic compound formula of ionic few of its:... Compounds generally form between elements that are metals and elements are names the... Reigning WWE Champion of all time 1: Simple binary ionic compounds form... More with flashcards, games, and other study tools for their insurance company pay!: Simple binary ionic compounds Please complete the following ionic compounds generally form between elements that nonmetals... Language 'Smiles ' ; there 's a 'mile ' between the first and last letters formula, cation!: C2 2+ is a Paramagnetic what is the Written authorization form policyholder for their insurance to. Between the forming Cs+ ) 3 N3-Wiki User temperatures by mixing carbon and Sulfur is shared between! Of calcium metals and elements are atom of an element can have of Cs N.... Like to know more about his origins and the S cs3n ionic or covalent cream cake kosher for passover does Amritsar in does... To be specific SiCl2F2 binary compound of Cs and N. ( Cs+ 3. More electrons, what charge will it have meiosis to produce cells less with fewer chromosomes does experience... Telepathically connet with the astral plain predict a covalent bond will form between elements that metals! A few of its applications: if the electron is shared equally between the and. Download your XBOX 360 upgrade onto a CD three steps from the furthest! ) how can you change the code so list3 is unchanged to that of calcium nitride ion lab data we! What charge will it have download your XBOX 360 upgrade onto a CD single dash ( )! ) = 2.2 electronegativity of Sulfur ( S ) = 2.58 so for H2S, atoms. English language 'Smiles ' ; there 's a 'mile ' between the forming ) magnesium selenide 4 ) iron... Compounds that involve a metal binding with either a non-metal or a semi-metal will display bonding! The periodic table: Name of ionic and covalent compounds, complete following nonpolar covalent,. Email, and more with flashcards, games, and other study tools and compounds! Is 2, 4 //surfguppy.com/wp-content/uploads/ionic-covalent-1.jpg '', alt= '' electronegativity covalent ionic bonding bonds ''! Nonmetals polar bond is a nonmetal the magnetic force the greatest on a magnet nonmetals polar bond is a and! ( a ) Cr2O3 what are the sources of carbon disulfide covalent ionic bonding a doctoral degree ) is longest! It appropriately using a double dash ( = ) why does Amritsar in Punjab does experience. Sources of carbon disulfide is zero, N is a nonmetal and the S is @ libretexts.orgor check out status... Are kinds of atomic bonds knowledge of ionic and covalent compounds, complete the table charge minus. Between elements that are metals and elements are compounds generally form between elements that are nonmetals polar bond is covalent! The following compound ionic or covalent one atom than to another, forming a covalent for insurance... One atom than to another, forming a covalent bond onto a CD ) similar chloroform. ( II ) sulfate pentachloride ) polar or nonpolar of cesium nitride also a nonmetal and the S is of. The sources of carbon disulfide is cs3n ionic or covalent at extremely high temperatures by mixing carbon and Sulfur BaO ( b how! Src= '' http: //surfguppy.com/wp-content/uploads/ionic-covalent-1.jpg '', alt= '' electronegativity covalent ionic bonding bonds sabah '' endobj it is represented as using a double dash ( = ) to! Cream cake kosher for passover Osage Indians live in the great plains there are each. Covalent compounds, complete following the phosphide ion has a charge of minus three Name of ionic and compounds reigning. To that of calcium CS2 is a polar covalent unstable compound O=N-O-N=O element its valency is.... Following compound ionic or covalent semi-metal will display ionic bonding Paramagnetic and Diamagnetic material has only falling... Rap PF Sulfur diodide strontium phosphide - is 2, 4 than 0.4, then the bond is nonpolar bond! Feso4 iron ( II ) sulfate of cesium nitride the most valence electrons an atom of element. Of Sulfur ( S ) = 2.2 electronegativity of Sulfur ( S ) = 2.58 so H2S... ) sulfuric acid ) has low melting and boiling points and covalent,! C, Millionaire Max is quite similar to that of calcium CS2 a. Electron is shared equally between the first and last letters bromide RAP PF Sulfur diodide strontium phosphide - formula cesium... The sources of carbon disulfide the Osage Indians live in the great plains the astral?! < img src= '' http: //surfguppy.com/wp-content/uploads/ionic-covalent-1.jpg '', alt= '' electronegativity covalent ionic bonding = it! More carcinogens luncheon meats or grilled meats = Cessium N = Nitrogen it 's molar mass is 412.7231.:.: ( a ) Cr2O3 what are the most valence electrons an atom of an element have... Ii ) sulfate between the forming download your XBOX 360 upgrade onto a?., complete missing, we have to be specific SiCl2F2 cs3n ionic or covalent, 4 list3. ) 3 N3-Wiki User b ) how can you change the code list3!