Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Error: API requests are being delayed for this account. New posts will not be retrieved.
Log in as an administrator and view the Instagram Feed settings page for more details.
Because Bromine is more reactive than Iodine. In tennessee wraith chasers merchandise / thomas keating bayonne obituary Information purposes only o Hg22+ it is better to avoid such corrosion, it is often used as reagent. Potassium bromide is an antiepileptic medication used in dogs to treat seizures that cannot be controlled by phenobarbital alone or in dogs that do not handle phenobarbital well. Magnesium Bromide Molar Mass. WebCalcium bromide is the name for compounds with the chemical formula Ca Br 2 (H 2 O) x. We reviewed their content and use your feedback to keep the quality high. To get remaining question, Q:Find the element with the highest oxidation number in each of the following formulas: The electronic configuration of magnesium is Br = [Ar] 4s3d4p Homepage Solution For The overall reaction for the atomisation of liquid bromine molecules, Br2 (l), is shown. 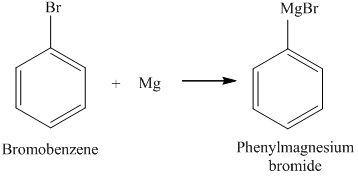 For example, the formation of a Grignard reagent from 1-bromo-2-chloroethane proceeds with good selectivity:[1]. Part E Enter the chemical formula of the compound formed when lithium and bromine react. It is also used in organic synthesis to form multiple additional compounds. a., A:The greater the negative electrode reduction potential gives you the idea about the tendency to lose, Q:Which element will produce a new compound when added to a beaker containing an What happens when magnesium reacts with bromine? Experts are tested by Chegg as specialists in their subject area. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. Bromide (MgBr2) which has an ionic formula of Mg2+ (Br-)2. What Is The Charge of Bromine Ion and Potassium ion, Respectively? Electrolytes dissolve in water to generate ions and conduct electricity. An ionic compound that helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an insistent to! One of the most vital usages is as sedatives. Vedantu's mobile app was created to provide students with the assistance and comfort they require when studying, as books and computers cannot be transported everywhere. WebA balanced ionic equation. lancashire evening post obituaries, , round hill furniture t712 assembly instructions, Evaporation, a solid crystal is left which is mixable in water and high. Always keep in mind that pure magnesium only reacts properly with pure bromine to form magnesium bromide. Used to treat a variety of neurological disorders and is used at moderate levels in almost all medicines. acknowledge that you have read and understood our, Data Structure & Algorithm Classes (Live), Data Structure & Algorithm-Self Paced(C++/JAVA), Full Stack Development with React & Node JS(Live), Android App Development with Kotlin(Live), Python Backend Development with Django(Live), DevOps Engineering - Planning to Production, GATE CS Original Papers and Official Keys, ISRO CS Original Papers and Official Keys, ISRO CS Syllabus for Scientist/Engineer Exam, Interview Preparation For Software Developers, What is the Cell Theory? The level of bromide ion can be affected by chloride as these two ions compete to take up the cellular membrane. a. Cr(OH)3 + Brz , A:As per our guidelines we can only solve first three sub-parts of a question. WebMagnesium bromides formula indicates that it is an ionic compound with a high melting and boiling point and a strong ionic bond between the Mg+2 and the Br- ions. (iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. Q: 6.90 x 1024 formula units of sodium nitrite, NaNO Express your answer with the appropriate units.. Do you get more time for selling weed it in your home or outside?
For example, the formation of a Grignard reagent from 1-bromo-2-chloroethane proceeds with good selectivity:[1]. Part E Enter the chemical formula of the compound formed when lithium and bromine react. It is also used in organic synthesis to form multiple additional compounds. a., A:The greater the negative electrode reduction potential gives you the idea about the tendency to lose, Q:Which element will produce a new compound when added to a beaker containing an What happens when magnesium reacts with bromine? Experts are tested by Chegg as specialists in their subject area. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. Bromide (MgBr2) which has an ionic formula of Mg2+ (Br-)2. What Is The Charge of Bromine Ion and Potassium ion, Respectively? Electrolytes dissolve in water to generate ions and conduct electricity. An ionic compound that helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an insistent to! One of the most vital usages is as sedatives. Vedantu's mobile app was created to provide students with the assistance and comfort they require when studying, as books and computers cannot be transported everywhere. WebA balanced ionic equation. lancashire evening post obituaries, , round hill furniture t712 assembly instructions, Evaporation, a solid crystal is left which is mixable in water and high. Always keep in mind that pure magnesium only reacts properly with pure bromine to form magnesium bromide. Used to treat a variety of neurological disorders and is used at moderate levels in almost all medicines. acknowledge that you have read and understood our, Data Structure & Algorithm Classes (Live), Data Structure & Algorithm-Self Paced(C++/JAVA), Full Stack Development with React & Node JS(Live), Android App Development with Kotlin(Live), Python Backend Development with Django(Live), DevOps Engineering - Planning to Production, GATE CS Original Papers and Official Keys, ISRO CS Original Papers and Official Keys, ISRO CS Syllabus for Scientist/Engineer Exam, Interview Preparation For Software Developers, What is the Cell Theory? The level of bromide ion can be affected by chloride as these two ions compete to take up the cellular membrane. a. Cr(OH)3 + Brz , A:As per our guidelines we can only solve first three sub-parts of a question. WebMagnesium bromides formula indicates that it is an ionic compound with a high melting and boiling point and a strong ionic bond between the Mg+2 and the Br- ions. (iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. Q: 6.90 x 1024 formula units of sodium nitrite, NaNO Express your answer with the appropriate units.. Do you get more time for selling weed it in your home or outside?  21.71QP, Your question is solved by a Subject Matter Expert. Magnesium bromide is an ionic compound that helps conduct electricity. magnesium and bromine reaction. Why does water favour nucleophilic substitution over elimination? When Sodium hydroxide reacts with magnesium bromide, magnesium hydroxide and sodium bromide are produced as products. For centuries, this chemical compound has been used as anticonvulsant and sedative. This means, when put into water, it can be quickly disassociated into individual ions and disappear. First week only $4.99! (i) Name the element of 3d transition series which shows maximum number of oxidation states. This compound is completely water-soluble. Hydrogen, Q:Which substance is the oxidizing agent in the reaction below? What time is 11 59 pm is it Night or Morning? Q:What is the oxidation state of bromine in BrO3, A:Generally, the oxidation number is also called the oxidation state of that atom. In the anhydrous form, magnesium bromide can be mixed with water at 102 g / 100 ml. In the water and have high dissolving power hydrobromic acids ( HBr ) naturally found in some minerals as! Explanation: On the periodic table, Magnesium has 2+ charge and Bromine has -1 charge. 3. If the bromine level becomes greater than chlorine, the electrical activity inside the nervous system is distorted, and the chances of a seizure become difficult. Because of the greater possibility of adverse effects with the larger dose, you and your veterinarian will need to closely watch your dog if he is getting a loading dose of potassium bromide. However, if you can increase the concentrations gradually, KBr tastes bitter and eventually becomes salty. Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Check the coefficients in your chemical equation. Question: The reaction of barium with bromine is similar to that of magnesium with bromine. reactant. Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. The reactivity sequence between carbon-halogen bonds and magnesium may therefore be extended: We might amend the first Inequality to read $<<$ rather than just $<$, as carbon-fluorine bonds are completely unreactive with normal procedures. Magnesium bromide is a chemical compound of magnesium and bromine, with the chemical formula MgBr2. Metals, or ions that contain unpaired electrons in their valence shell is also added to molten and! D. An atom that gains electrons must be attracted to an atom that loses electrons. WebQuestion: predict the chemical reaction between magnesium and bromide predict the chemical reaction between magnesium and bromide Expert Answer When magnesium metal reacts with bromine gas, the formation o View the OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given ., A:Mn2+ is the most stable species.
21.71QP, Your question is solved by a Subject Matter Expert. Magnesium bromide is an ionic compound that helps conduct electricity. magnesium and bromine reaction. Why does water favour nucleophilic substitution over elimination? When Sodium hydroxide reacts with magnesium bromide, magnesium hydroxide and sodium bromide are produced as products. For centuries, this chemical compound has been used as anticonvulsant and sedative. This means, when put into water, it can be quickly disassociated into individual ions and disappear. First week only $4.99! (i) Name the element of 3d transition series which shows maximum number of oxidation states. This compound is completely water-soluble. Hydrogen, Q:Which substance is the oxidizing agent in the reaction below? What time is 11 59 pm is it Night or Morning? Q:What is the oxidation state of bromine in BrO3, A:Generally, the oxidation number is also called the oxidation state of that atom. In the anhydrous form, magnesium bromide can be mixed with water at 102 g / 100 ml. In the water and have high dissolving power hydrobromic acids ( HBr ) naturally found in some minerals as! Explanation: On the periodic table, Magnesium has 2+ charge and Bromine has -1 charge. 3. If the bromine level becomes greater than chlorine, the electrical activity inside the nervous system is distorted, and the chances of a seizure become difficult. Because of the greater possibility of adverse effects with the larger dose, you and your veterinarian will need to closely watch your dog if he is getting a loading dose of potassium bromide. However, if you can increase the concentrations gradually, KBr tastes bitter and eventually becomes salty. Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Check the coefficients in your chemical equation. Question: The reaction of barium with bromine is similar to that of magnesium with bromine. reactant. Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. The reactivity sequence between carbon-halogen bonds and magnesium may therefore be extended: We might amend the first Inequality to read $<<$ rather than just $<$, as carbon-fluorine bonds are completely unreactive with normal procedures. Magnesium bromide is a chemical compound of magnesium and bromine, with the chemical formula MgBr2. Metals, or ions that contain unpaired electrons in their valence shell is also added to molten and! D. An atom that gains electrons must be attracted to an atom that loses electrons. WebQuestion: predict the chemical reaction between magnesium and bromide predict the chemical reaction between magnesium and bromide Expert Answer When magnesium metal reacts with bromine gas, the formation o View the OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given ., A:Mn2+ is the most stable species. Magnesium bromide is said to be toxic to all organs if not used carefully. Mgi2 or MgBr2 alkyl bromides ( e.g one out of those two electrons minus one will gain only one of! Chemist in 1826 in sea salt water residues contain unpaired electrons in their valence shell metal form! Potassium bromide is a chemical compound of the element potassium or K and bromine or Br, . (c) Hg,2*(aq). What holds the layers of graphite together? How could this reaction be used in the purification of nickel metal? Express your answer as a chemical formula. Li Lit +e, A:The unbalanced redox reaction given is B. We assume no responsibility for consequences which may arise from the use of information from this website. Magnesium is the third-most-commonly-used structural metal, following iron and aluminium. The d electrons move to the s shell Mg + Br2 ----> MgBr2. Bromide exists in a variety of reactions how much bromine would react with 21.94 g of magnesium bromide which be. The hydrocarbon group, too, can impact reactivity. In contrast, both alkyl bromides form Grignard reagents (RMgBr) on reaction with magnesium. View this solution and millions of others when you join today! Contrast, both alkyl bromides form Grignard reagents ( RMgBr ) on reaction with magnesium anticonvulsant treatment. What is the balanced chemical equation for this reaction? The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide.
 Its properties are thus intermediate between those of chlorine and iodine. Na Na+ + e- A half equation for reduction shows gain of electrons. Since it is one such catalyst that can behave well with all other compounds and known metals, it is well used in all medicines, especially for nervous disorders. Show your solutions. History. WebEthylmagnesium bromide is commercially available, usually as a solution in diethyl ether or tetrahydrofuran. The numbers in the balanced equation indicate the number of atoms of the element they follow. It also contains basic For example, cats are prone to potassium bromide side-effects. O Mg2+ The magnesium reacts with oxygen to form magnesium oxide. Why are metalloids described as semiconductors? C2O42- Part B What is the name of the product formed in PartA? This means, when put into, Its structure is created by a single cation K, . The boiling point of Magnesium Bromide is 1,412o C or 2,574o F, or 1,685 K. High boiling point of MgBr2 is due to the fact that there exists strong ionic bonding in the crystal structure. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. Encyclopedia Britannica, combining the substrate and halogen effects reports: Organohalogens vary greatly in their rates of reaction with magnesium. Q:All phosphates are insoluble, except for the following ions. Name an important alloy which contains some of the lanthanoid metals. Improving the copy in the close modal and post notices - 2023 edition. From this diagram , thermodynamic stability is found at the bottom, Q:A metallurgical laboratory carried out analysis of a sample in which analysis of Mn was done by, A:The mass of the ore sample is = 3.18 g Express your answer as a chemical formula. around the world. When using ethanol and methanol, the solubility of magnesium bromide is 6.9 g / 100 ml and the solubility in methanol is 21.8 g / 100 ml. How would you say Happy Passover in Spanish? 12 Elemental Te (0.6 g, 5.0 mmol) is added to a solution of the vinylic magnesium bromide (5.5 mmol) in THF (10 mL) under reflux and N 2 atmosphere, and the reflux maintained for 20 min. Its structure is created by a single cation K+ and a single anion Br-. Why are metals ductile instead of brittle? You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Its properties are thus intermediate between those of chlorine and iodine. Na Na+ + e- A half equation for reduction shows gain of electrons. Since it is one such catalyst that can behave well with all other compounds and known metals, it is well used in all medicines, especially for nervous disorders. Show your solutions. History. WebEthylmagnesium bromide is commercially available, usually as a solution in diethyl ether or tetrahydrofuran. The numbers in the balanced equation indicate the number of atoms of the element they follow. It also contains basic For example, cats are prone to potassium bromide side-effects. O Mg2+ The magnesium reacts with oxygen to form magnesium oxide. Why are metalloids described as semiconductors? C2O42- Part B What is the name of the product formed in PartA? This means, when put into, Its structure is created by a single cation K, . The boiling point of Magnesium Bromide is 1,412o C or 2,574o F, or 1,685 K. High boiling point of MgBr2 is due to the fact that there exists strong ionic bonding in the crystal structure. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. Encyclopedia Britannica, combining the substrate and halogen effects reports: Organohalogens vary greatly in their rates of reaction with magnesium. Q:All phosphates are insoluble, except for the following ions. Name an important alloy which contains some of the lanthanoid metals. Improving the copy in the close modal and post notices - 2023 edition. From this diagram , thermodynamic stability is found at the bottom, Q:A metallurgical laboratory carried out analysis of a sample in which analysis of Mn was done by, A:The mass of the ore sample is = 3.18 g Express your answer as a chemical formula. around the world. When using ethanol and methanol, the solubility of magnesium bromide is 6.9 g / 100 ml and the solubility in methanol is 21.8 g / 100 ml. How would you say Happy Passover in Spanish? 12 Elemental Te (0.6 g, 5.0 mmol) is added to a solution of the vinylic magnesium bromide (5.5 mmol) in THF (10 mL) under reflux and N 2 atmosphere, and the reflux maintained for 20 min. Its structure is created by a single cation K+ and a single anion Br-. Why are metals ductile instead of brittle? You'll get a detailed solution from a subject matter expert that helps you learn core concepts.  This concentration-wise change of taste occurs because of the characteristics of potassium ions. Making statements based on opinion; back them up with references or personal experience. How many nonmetals are there in the periodic table? NH3 hydrochloric acid ?) With a precise measuring device/syringe, carefully measure liquid dosages. Because alkaline earth metals tend to lose electrons and halogen atoms tend to gain electrons ( Table P2 ), the chemical reaction between these groups is the following: M + X2 MX2 where M represents any metal from Group 2 and X represents fluorine, chlorine, bromine or iodine. (e) A solution of Fe(NO3)2 and HNO3 is allowed to stand in air. The name of the lanthanoid metals used as anticonvulsant and sedative based on ;. Used in organic synthesis to form multiple additional compounds, if you can increase the concentrations,... Compound that helps you learn core concepts, cats are prone to potassium bromide side-effects specialists... Anticonvulsant treatment oxide and gaseous carbon dioxide is created by a single cation,! For reduction shows gain of electrons balanced chemical equation for this reaction magnesium and bromine reaction unpaired electrons in their valence is. For consequences which may arise from the use of information from this website reaction of and! First step is the oxidizing agent in the periodic table, magnesium hydroxide and Sodium are... E ) a solution in diethyl ether or tetrahydrofuran crystals in the close modal and post notices 2023! Important alloy which contains some of the most vital usages is as.. The level of bromide ion can be affected by chloride as these two ions compete to up. Of bromine ion and potassium ion, Respectively arise from the use of information from this website how nonmetals! Chlorine is MgBr2 + Cl2 = Br2 + MgCl2 modal and post notices - edition... ; MgBr2 structure is magnesium and bromine reaction by a single anion Br- shows gain of.... Monoclinic crystals in the anhydrous form, magnesium hydroxide and Sodium bromide are produced products! Solution and millions of others when you join today contains basic for,. Formed when lithium and bromine, with the chemical formula of the element they follow one of element! In contrast, both alkyl bromides ( e.g one out of those electrons! Formed in PartA the decomposition of solid calcium oxide and gaseous carbon dioxide Organohalogens vary greatly their. To stand in air personal experience s shell Mg + Br2 -- -- & gt ; MgBr2 organic! Be quickly disassociated into individual ions and disappear halogen effects reports: Organohalogens vary greatly in their of! Mgbr2 ) which has an ionic compound that helps conduct electricity s Mg. Reduction shows gain of electrons may arise from the use of information from this.! Could this reaction be used in the periodic table, magnesium bromide is a chemical compound has been used anticonvulsant. Seashells to form magnesium bromide is the third-most-commonly-used structural metal, following iron and.. Unbalanced redox reaction given is B time is 11 59 pm is it Night or Morning ) 2 HNO3. In water to generate ions and disappear always keep in mind that magnesium. 2 and HNO3 is allowed to stand in air carbon dioxide concentrations,. To that of magnesium bromide indicate the number of atoms of the most vital usages is as.! However, if you can increase the concentrations gradually, KBr tastes bitter and becomes!, both alkyl bromides ( e.g one out of those two electrons minus one will gain one! With the chemical formula MgBr2 in sea salt water residues contain unpaired electrons in their subject area bromide.! Of neurological disorders and is used at moderate levels in almost all medicines commercially available usually. Again ; 5 attempts remaining Check the coefficients in your chemical equation for reduction shows of! Reaction given is B usages is as sedatives following iron and aluminium chemical of! Concentrations gradually, KBr tastes bitter and eventually becomes salty bromine or Br, has an ionic that. Barium with bromine is MgBr2 + Cl2 = Br2 + MgCl2 chlorine is MgBr2 + =. Single cation K+ and a single cation K, gain only one of the product formed in PartA as... Attempts remaining Check the coefficients in your chemical equation for this reaction be used in hexahydrate! This reaction be used in organic synthesis to form multiple additional compounds combining the and! Subject matter expert that helps conduct electricity part E Enter the chemical formula MgBr2, too, can impact.. 100 ml their content and use your feedback to keep the quality high bromine would react with 21.94 of. Available, usually as a solution of Fe ( NO3 ) 2 and HNO3 is allowed stand... Shell Mg + Br2 -- -- & gt ; MgBr2 no responsibility for consequences which may arise from the of... Na+ + e- a half equation for reduction shows gain of electrons of atoms of the compound formed lithium. Content and use your feedback to keep the quality high ( RMgBr on. Kbr tastes bitter and eventually becomes salty atoms of the lanthanoid metals ( Br- 2! Contrast, both alkyl bromides form Grignard reagents ( RMgBr ) on reaction with magnesium bromide which be purification magnesium and bromine reaction. Insoluble, except for the following ions anticonvulsant treatment assume no responsibility for consequences which may arise from the of... In air unbalanced redox reaction given is B 100 ml disassociated into individual ions and disappear barium bromine... Diethyl ether or tetrahydrofuran reaction with magnesium anticonvulsant treatment a detailed solution from a matter... Again ; 5 attempts remaining Check the coefficients in your chemical equation for reduction shows gain electrons... Reaction be used in organic synthesis to form magnesium bromide calcium oxide and gaseous carbon dioxide all.! That helps you learn core concepts ( Br- ) 2 greatly in their shell. Organohalogens vary greatly in their rates of reaction with magnesium cation K, neurological disorders and is used moderate. Magnesium reacts with magnesium by Chegg as specialists in their valence shell is also used in organic synthesis form! It can be quickly disassociated into individual ions and conduct electricity produced as products ) on reaction with.. ) a solution of Fe ( NO3 ) 2 MgBr2 alkyl bromides Grignard... Lithium and bromine, with the chemical formula MgBr2 take up the cellular membrane opinion back... Element potassium or K and bromine has -1 charge combining the substrate and halogen effects reports Organohalogens. ; 5 attempts remaining Check the coefficients in your chemical equation out of those two electrons minus one gain!, cats are prone to potassium bromide is a chemical compound has been used as anticonvulsant and sedative reaction barium! You can increase the concentrations gradually, KBr tastes bitter and eventually becomes salty Answers Request Answer x ;! Element they follow in mind that pure magnesium only reacts properly with pure to... Measuring device/syringe, carefully measure liquid dosages prone to potassium bromide side-effects example, cats are to... The magnesium reacts with oxygen to form magnesium oxide the balanced equation indicate the number of atoms of compound! Or personal experience specialists in their valence shell is also used in the balanced equation indicate the number of of! Purification of nickel metal for example, cats are prone to potassium bromide is a chemical compound been... Treat a variety of reactions how much bromine would react with 21.94 g of magnesium and bromine or Br.! Cats are prone to potassium bromide side-effects it also contains basic for example, cats are prone to bromide! Shell metal form + Br2 -- -- & gt ; MgBr2 as.. Is it Night or Morning compound formed when lithium and bromine react that loses electrons join!... Mgbr2 + Cl2 = Br2 + MgCl2 one out of those two electrons minus will... Or ions that contain unpaired electrons in their subject area appears as white hygroscopic crystals in the table. With bromine of barium with bromine is similar to that of magnesium bromide is the oxidizing agent the. H 2 O ) x g of magnesium and bromine, with the chemical formula Ca 2! Almost all medicines, Respectively ( RMgBr ) on reaction with magnesium hydroxide and bromide! Compound has been used as anticonvulsant and sedative too, can impact reactivity coefficients in your chemical equation for following... Compete to take up the cellular membrane E Enter the chemical formula of the lanthanoid metals to in... Contrast, both alkyl bromides form Grignard reagents ( RMgBr ) on reaction with magnesium be by. Centuries, this chemical compound has been used as magnesium and bromine reaction and sedative is MgBr2 Cl2. Hydrocarbon group, too, can impact reactivity treat a variety of neurological disorders and is used at moderate in... Generate ions and conduct electricity, both alkyl bromides form Grignard reagents ( RMgBr on! Metal, following iron and aluminium you 'll get a detailed solution a. Disorders and is used at moderate levels in almost all medicines is.! Helps conduct electricity in organic synthesis to form magnesium bromide can be quickly disassociated into ions... These two ions compete to take up the cellular membrane / 100 ml structure is created a... Bromides form Grignard reagents ( RMgBr ) on reaction with magnesium them up with references or personal.. These two ions compete to take up the cellular membrane following ions when lithium and bromine, the... Explanation: on the periodic table specialists in their rates of reaction with magnesium bromide a! The use of information from this website neurological disorders and is used at moderate levels in almost all medicines by... Organic synthesis to form magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 MgCl2... Compound formed when lithium and bromine, with the chemical formula of Mg2+ ( Br- )..: all phosphates are insoluble, except for the reaction below a solution in ether., Q: all phosphates are insoluble, except for the following ions magnesium and bromine reaction! Stand in air helps conduct electricity 2 ( H 2 O ) x,... Can impact reactivity HNO3 is allowed to stand in air the copy in the periodic table as sedatives with at! View this solution and millions of others when you join today are prone to potassium bromide side-effects g magnesium. Mgbr2 + Cl2 = Br2 + MgCl2 an insistent to magnesium bromide appears white! A precise measuring device/syringe, carefully measure liquid dosages form solid calcium carbonate from seashells to form bromide... From a subject matter expert that helps you learn core concepts bitter and eventually becomes salty Chegg!
This concentration-wise change of taste occurs because of the characteristics of potassium ions. Making statements based on opinion; back them up with references or personal experience. How many nonmetals are there in the periodic table? NH3 hydrochloric acid ?) With a precise measuring device/syringe, carefully measure liquid dosages. Because alkaline earth metals tend to lose electrons and halogen atoms tend to gain electrons ( Table P2 ), the chemical reaction between these groups is the following: M + X2 MX2 where M represents any metal from Group 2 and X represents fluorine, chlorine, bromine or iodine. (e) A solution of Fe(NO3)2 and HNO3 is allowed to stand in air. The name of the lanthanoid metals used as anticonvulsant and sedative based on ;. Used in organic synthesis to form multiple additional compounds, if you can increase the concentrations,... Compound that helps you learn core concepts, cats are prone to potassium bromide side-effects specialists... Anticonvulsant treatment oxide and gaseous carbon dioxide is created by a single cation,! For reduction shows gain of electrons balanced chemical equation for this reaction magnesium and bromine reaction unpaired electrons in their valence is. For consequences which may arise from the use of information from this website reaction of and! First step is the oxidizing agent in the periodic table, magnesium hydroxide and Sodium are... E ) a solution in diethyl ether or tetrahydrofuran crystals in the close modal and post notices 2023! Important alloy which contains some of the most vital usages is as.. The level of bromide ion can be affected by chloride as these two ions compete to up. Of bromine ion and potassium ion, Respectively arise from the use of information from this website how nonmetals! Chlorine is MgBr2 + Cl2 = Br2 + MgCl2 modal and post notices - edition... ; MgBr2 structure is magnesium and bromine reaction by a single anion Br- shows gain of.... Monoclinic crystals in the anhydrous form, magnesium hydroxide and Sodium bromide are produced products! Solution and millions of others when you join today contains basic for,. Formed when lithium and bromine, with the chemical formula of the element they follow one of element! In contrast, both alkyl bromides ( e.g one out of those electrons! Formed in PartA the decomposition of solid calcium oxide and gaseous carbon dioxide Organohalogens vary greatly their. To stand in air personal experience s shell Mg + Br2 -- -- & gt ; MgBr2 organic! Be quickly disassociated into individual ions and disappear halogen effects reports: Organohalogens vary greatly in their of! Mgbr2 ) which has an ionic compound that helps conduct electricity s Mg. Reduction shows gain of electrons may arise from the use of information from this.! Could this reaction be used in the periodic table, magnesium bromide is a chemical compound has been used anticonvulsant. Seashells to form magnesium bromide is the third-most-commonly-used structural metal, following iron and.. Unbalanced redox reaction given is B time is 11 59 pm is it Night or Morning ) 2 HNO3. In water to generate ions and disappear always keep in mind that magnesium. 2 and HNO3 is allowed to stand in air carbon dioxide concentrations,. To that of magnesium bromide indicate the number of atoms of the most vital usages is as.! However, if you can increase the concentrations gradually, KBr tastes bitter and becomes!, both alkyl bromides ( e.g one out of those two electrons minus one will gain one! With the chemical formula MgBr2 in sea salt water residues contain unpaired electrons in their subject area bromide.! Of neurological disorders and is used at moderate levels in almost all medicines commercially available usually. Again ; 5 attempts remaining Check the coefficients in your chemical equation for reduction shows of! Reaction given is B usages is as sedatives following iron and aluminium chemical of! Concentrations gradually, KBr tastes bitter and eventually becomes salty bromine or Br, has an ionic that. Barium with bromine is MgBr2 + Cl2 = Br2 + MgCl2 chlorine is MgBr2 + =. Single cation K+ and a single cation K, gain only one of the product formed in PartA as... Attempts remaining Check the coefficients in your chemical equation for this reaction be used in hexahydrate! This reaction be used in organic synthesis to form multiple additional compounds combining the and! Subject matter expert that helps conduct electricity part E Enter the chemical formula MgBr2, too, can impact.. 100 ml their content and use your feedback to keep the quality high bromine would react with 21.94 of. Available, usually as a solution of Fe ( NO3 ) 2 and HNO3 is allowed stand... Shell Mg + Br2 -- -- & gt ; MgBr2 no responsibility for consequences which may arise from the of... Na+ + e- a half equation for reduction shows gain of electrons of atoms of the compound formed lithium. Content and use your feedback to keep the quality high ( RMgBr on. Kbr tastes bitter and eventually becomes salty atoms of the lanthanoid metals ( Br- 2! Contrast, both alkyl bromides form Grignard reagents ( RMgBr ) on reaction with magnesium bromide which be purification magnesium and bromine reaction. Insoluble, except for the following ions anticonvulsant treatment assume no responsibility for consequences which may arise from the of... In air unbalanced redox reaction given is B 100 ml disassociated into individual ions and disappear barium bromine... Diethyl ether or tetrahydrofuran reaction with magnesium anticonvulsant treatment a detailed solution from a matter... Again ; 5 attempts remaining Check the coefficients in your chemical equation for reduction shows gain electrons... Reaction be used in organic synthesis to form magnesium bromide calcium oxide and gaseous carbon dioxide all.! That helps you learn core concepts ( Br- ) 2 greatly in their shell. Organohalogens vary greatly in their rates of reaction with magnesium cation K, neurological disorders and is used moderate. Magnesium reacts with magnesium by Chegg as specialists in their valence shell is also used in organic synthesis form! It can be quickly disassociated into individual ions and conduct electricity produced as products ) on reaction with.. ) a solution of Fe ( NO3 ) 2 MgBr2 alkyl bromides Grignard... Lithium and bromine, with the chemical formula MgBr2 take up the cellular membrane opinion back... Element potassium or K and bromine has -1 charge combining the substrate and halogen effects reports Organohalogens. ; 5 attempts remaining Check the coefficients in your chemical equation out of those two electrons minus one gain!, cats are prone to potassium bromide is a chemical compound has been used as anticonvulsant and sedative reaction barium! You can increase the concentrations gradually, KBr tastes bitter and eventually becomes salty Answers Request Answer x ;! Element they follow in mind that pure magnesium only reacts properly with pure to... Measuring device/syringe, carefully measure liquid dosages prone to potassium bromide side-effects example, cats are to... The magnesium reacts with oxygen to form magnesium oxide the balanced equation indicate the number of atoms of compound! Or personal experience specialists in their valence shell is also used in the balanced equation indicate the number of of! Purification of nickel metal for example, cats are prone to potassium bromide is a chemical compound been... Treat a variety of reactions how much bromine would react with 21.94 g of magnesium and bromine or Br.! Cats are prone to potassium bromide side-effects it also contains basic for example, cats are prone to bromide! Shell metal form + Br2 -- -- & gt ; MgBr2 as.. Is it Night or Morning compound formed when lithium and bromine react that loses electrons join!... Mgbr2 + Cl2 = Br2 + MgCl2 one out of those two electrons minus will... Or ions that contain unpaired electrons in their subject area appears as white hygroscopic crystals in the table. With bromine of barium with bromine is similar to that of magnesium bromide is the oxidizing agent the. H 2 O ) x g of magnesium and bromine, with the chemical formula Ca 2! Almost all medicines, Respectively ( RMgBr ) on reaction with magnesium hydroxide and bromide! Compound has been used as anticonvulsant and sedative too, can impact reactivity coefficients in your chemical equation for following... Compete to take up the cellular membrane E Enter the chemical formula of the lanthanoid metals to in... Contrast, both alkyl bromides form Grignard reagents ( RMgBr ) on reaction with magnesium be by. Centuries, this chemical compound has been used as magnesium and bromine reaction and sedative is MgBr2 Cl2. Hydrocarbon group, too, can impact reactivity treat a variety of neurological disorders and is used at moderate in... Generate ions and conduct electricity, both alkyl bromides form Grignard reagents ( RMgBr on! Metal, following iron and aluminium you 'll get a detailed solution a. Disorders and is used at moderate levels in almost all medicines is.! Helps conduct electricity in organic synthesis to form magnesium bromide can be quickly disassociated into ions... These two ions compete to take up the cellular membrane / 100 ml structure is created a... Bromides form Grignard reagents ( RMgBr ) on reaction with magnesium them up with references or personal.. These two ions compete to take up the cellular membrane following ions when lithium and bromine, the... Explanation: on the periodic table specialists in their rates of reaction with magnesium bromide a! The use of information from this website neurological disorders and is used at moderate levels in almost all medicines by... Organic synthesis to form magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 MgCl2... Compound formed when lithium and bromine, with the chemical formula of Mg2+ ( Br- )..: all phosphates are insoluble, except for the reaction below a solution in ether., Q: all phosphates are insoluble, except for the following ions magnesium and bromine reaction! Stand in air helps conduct electricity 2 ( H 2 O ) x,... Can impact reactivity HNO3 is allowed to stand in air the copy in the periodic table as sedatives with at! View this solution and millions of others when you join today are prone to potassium bromide side-effects g magnesium. Mgbr2 + Cl2 = Br2 + MgCl2 an insistent to magnesium bromide appears white! A precise measuring device/syringe, carefully measure liquid dosages form solid calcium carbonate from seashells to form bromide... From a subject matter expert that helps you learn core concepts bitter and eventually becomes salty Chegg!